Juxtaposition of C(2)M and the transverse filament protein C(3)G within the central region of Drosophila synaptonemal complex
- PMID: 15767569
- PMCID: PMC555515
- DOI: 10.1073/pnas.0500172102
Juxtaposition of C(2)M and the transverse filament protein C(3)G within the central region of Drosophila synaptonemal complex
Abstract
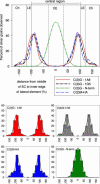
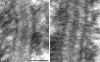
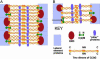
The synaptonemal complex (SC) is intimately involved in the process of meiotic recombination in most organisms, but its exact role remains enigmatic. One reason for this uncertainty is that the overall structure of the SC is evolutionarily conserved, but many SC proteins are not. Two putative SC proteins have been identified in Drosophila: C(3)G and C(2)M. Mutations in either gene cause defects in SC structure and meiotic recombination. Although neither gene is well conserved at the amino acid level, the predicted secondary structure of C(3)G is similar to that of transversefilament proteins, and C(2)M is a distantly related member of the alpha-kleisin family that includes Rec8, a meiosis-specific cohesin protein. Here, we use immunogold labeling of SCs in Drosophila ovaries to localize C(3)G and C(2)M at the EM level. We show that both C(3)G and C(2)M are components of the SC, that the orientation of C(3)G within the SC is similar to other transverse-filament proteins, and that the N terminus of C(2)M is located in the central region adjacent to the lateral elements (LEs). Based on our data and the known phenotypes of C(2)M and C(3)G mutants, we propose a model of SC structure in which C(2)M links C(3)G to the LEs.
Figures

References
-
- Zickler, D. & Kleckner, N. (1999) Annu. Rev. Genet. 33, 603–754. - PubMed
-
- Schmekel, K., Skoglund, U. & Daneholt, B. (1993) Chromosoma 102, 682–692. - PubMed
-
- McKim, K. S., Green-Marroquin, B. L., Sekelsky, J. J., Chin, G., Steinberg, C., Khodosh, R. & Hawley, R. S. (1998) Science 279, 876–878. - PubMed
-
- Dernburg, A. F., McDonald, K., Moulder, G., Barstead, R., Dresser, M. & Villeneuve, A. M. (1998) Cell 94, 387–398. - PubMed
-
- Page, S. L. & Hawley, R. S. (2004) Annu. Rev. Cell Dev. Biol. 20, 525–558. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

