Evidence for cross-genotype neutralization of hepatitis C virus pseudo-particles and enhancement of infectivity by apolipoprotein C1
- PMID: 15767578
- PMCID: PMC555507
- DOI: 10.1073/pnas.0501275102
Evidence for cross-genotype neutralization of hepatitis C virus pseudo-particles and enhancement of infectivity by apolipoprotein C1
Abstract
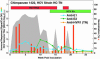
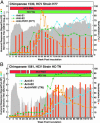
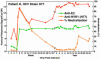
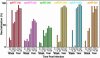
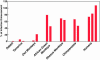
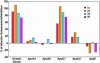
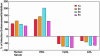
The lack of a cell culture system to support hepatitis C virus (HCV) replication has hampered studies of this frequent cause of chronic liver disease. However, pseudotyped retroviral particles (pp) bearing the HCV envelope glycoproteins have provided a different approach to HCV studies. We used genotype 1a pp to detect neutralizing antibodies (NtAb) in eight chimpanzees and four humans infected with 1a strains, and developed pp of genotypes 2a, 3a, 4a, 5a, and 6a to study crossreactivity. NtAb was detected in one of four chimpanzees and none of three humans with acute resolving infection, suggesting that NtAb is not required for HCV clearance. NtAb were detected at high titer in two of four chimpanzees and, in Patient H, all with persistent infection; responses paralleled humoral responses to envelope 1 and 2 proteins and, in some cases, correlate also with antibodies to the hypervariable region 1, previously thought to be the primary site of neutralization. NtAb raised during 1a infections could neutralize HCVpp of genotypes 4a, 5a, and 6a but had only limited reactivity against 2a and 3a. The detection of high-titer NtAb with cross-genotype reactivity has important implications for the development of active and passive immune-prophylaxis strategies against HCV. Finally, we found that HCVpp infectivity was enhanced by human or chimpanzee sera; apolipoprotein C1 alone or as a component of high-density lipoproteins caused this enhancement. Future studies of the in vivo role of apolipoprotein C1 might provide additional insights into the infection process of HCV.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

