Viable adenovirus vaccine prototypes: high-level production of a papillomavirus capsid antigen from the major late transcriptional unit
- PMID: 15767581
- PMCID: PMC554749
- DOI: 10.1073/pnas.0500933102
Viable adenovirus vaccine prototypes: high-level production of a papillomavirus capsid antigen from the major late transcriptional unit
Abstract
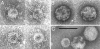
Safe, effective, orally delivered, live adenovirus vaccines have been in use for three decades. Recombinant derivatives of the live adenovirus vaccines may prove an economical alternative to current vaccines for a variety of diseases. To explore that possibility, we constructed a series of recombinants that express the major capsid protein (L1) of canine oral papillomavirus (COPV), a model for mucosal human papillomavirus (HPV) infection. Vaccination with virus-like particles (VLPs) composed of recombinant HPV L1 completely prevents persistent HPV infection [Koutsky, L. A., Ault, K. A., Wheeler, C. M., Brown, D. R., Barr, E., Alvarez, F. B., Chiacchierini, L. M. & Jansen, K. U. (2002) N. Engl. J. Med. 347, 1645-1651], suggesting that L1 expressed from recombinant adenoviruses might provide protective immunity. In our recombinants, COPV L1 is incorporated into adenovirus late region 5 (Ad L5) and is expressed as a member of the adenoviral major late transcriptional unit (MLTU). COPV L1 production by the most prolific recombinant is comparable to that of the most abundant adenoviral protein, hexon. COPV L1 production by recombinants is influenced by Ad L5 gene order, the specific mRNA processing signals associated with COPV L1, and the state of a putative splicing inhibitor in the COPV L1 gene. Recombinant COPV L1 protein assembles into VLPs that react with an antibody specific for conformational epitopes on native COPV L1 protein that correlate with protection in vivo. The designs of these recombinants can be applied directly to the production of recombinants appropriate for assessing immunogenicity and protective efficacy in animal models and in human trials.
Figures


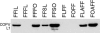

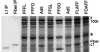
Similar articles
-
Mutant canine oral papillomavirus L1 capsid proteins which form virus-like particles but lack native conformational epitopes.J Gen Virol. 1998 Sep;79 ( Pt 9):2137-46. doi: 10.1099/0022-1317-79-9-2137. J Gen Virol. 1998. PMID: 9747722
-
Protection of beagle dogs from mucosal challenge with canine oral papillomavirus by immunization with recombinant adenoviruses expressing codon-optimized early genes.Virology. 2005 Jun 5;336(2):208-18. doi: 10.1016/j.virol.2005.03.022. Virology. 2005. PMID: 15892962
-
HPV16 L1 capsid protein expressed from viable adenovirus recombinants elicits neutralizing antibody in mice.Vaccine. 2007 Apr 30;25(17):3501-10. doi: 10.1016/j.vaccine.2006.06.080. Epub 2006 Jul 18. Vaccine. 2007. PMID: 16914239
-
Human papillomavirus vaccines.Rev Med Virol. 2006 May-Jun;16(3):139-49. doi: 10.1002/rmv.498. Rev Med Virol. 2006. PMID: 16710836 Review.
-
Progress in prophylactic and therapeutic vaccines for human papillomavirus infection.Expert Rev Vaccines. 2003 Jun;2(3):381-9. doi: 10.1586/14760584.2.3.381. Expert Rev Vaccines. 2003. PMID: 12903803 Review.
Cited by
-
Adenovirus particles that display the Plasmodium falciparum circumsporozoite protein NANP repeat induce sporozoite-neutralizing antibodies in mice.Vaccine. 2011 Feb 11;29(8):1683-9. doi: 10.1016/j.vaccine.2010.12.040. Epub 2011 Jan 1. Vaccine. 2011. PMID: 21199707 Free PMC article.
-
Regulation of human papillomavirus gene expression by splicing and polyadenylation.Nat Rev Microbiol. 2013 Apr;11(4):239-51. doi: 10.1038/nrmicro2984. Epub 2013 Mar 11. Nat Rev Microbiol. 2013. PMID: 23474685 Review.
-
Immune responses in macaques to a prototype recombinant adenovirus live oral human papillomavirus 16 vaccine.Clin Vaccine Immunol. 2014 Sep;21(9):1224-31. doi: 10.1128/CVI.00197-14. Epub 2014 Jul 2. Clin Vaccine Immunol. 2014. PMID: 24990902 Free PMC article.
-
Cervical human papillomavirus infection and squamous intraepithelial lesions in rural Gambia, West Africa: viral sequence analysis and epidemiology.Br J Cancer. 2005 Oct 31;93(9):1068-76. doi: 10.1038/sj.bjc.6602736. Br J Cancer. 2005. PMID: 16106268 Free PMC article.
-
Applying genomic and bioinformatic resources to human adenovirus genomes for use in vaccine development and for applications in vector development for gene delivery.Viruses. 2010 Jan;2(1):1-26. doi: 10.3390/v2010001. Epub 2010 Jan 6. Viruses. 2010. PMID: 21994597 Free PMC article.
References
-
- Gaydos, C. A. & Gaydos, J. C. (2004) in Vaccines, eds. Plotkin, S. A. & Orenstein, M. D. (Saunders, Philadelphia), pp. 863–885.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

