Deficits in motor coordination with aberrant cerebellar development in mice lacking testicular orphan nuclear receptor 4
- PMID: 15767677
- PMCID: PMC1061629
- DOI: 10.1128/MCB.25.7.2722-2732.2005
Deficits in motor coordination with aberrant cerebellar development in mice lacking testicular orphan nuclear receptor 4
Abstract
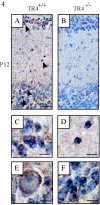
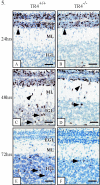
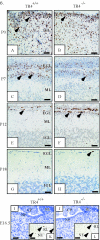
Since testicular orphan nuclear receptor 4 (TR4) was cloned, its physiological function has remained largely unknown. Throughout postnatal development, TR4-knockout (TR4-/-) mice exhibited behavioral deficits in motor coordination, suggesting impaired cerebellar function. Histological examination of the postnatal TR4-/- cerebellum revealed gross abnormalities in foliation; specifically, lobule VII in the anterior vermis was missing. Further analyses demonstrated that the laminations of the TR4-/- cerebellar cortex were changed, including reductions in the thickness of the molecular layer and the internal granule layer, as well as delayed disappearance of the external granule cell layer (EGL). These lamination irregularities may result from interference with granule cell proliferation within the EGL, delayed inward migration of postmitotic granule cells, and a higher incidence of apoptotis. In addition, abnormal development of Purkinje cells was observed in the postnatal TR4-/- cerebellum, as evidenced by aberrant dendritic arborization and reduced calbindin staining intensity. Expression of Pax-6, Sonic Hedgehog (Shh), astrotactin (Astn), reelin, and Cdk-5, genes correlated with the morphological development of the cerebellum, is reduced in the developing TR4-/- cerebellum. Together, our findings suggest that TR4 is required for normal cerebellar development.
Figures





References
-
- Adams, N. C., T. Tomoda, M. Cooper, G. Dietz, and M. E. Hatten. 2002. Mice that lack astrotactin have slowed neuronal migration. Development 129:965-972. - PubMed
-
- Bock, H. H., Y. Jossin, P. May, O. Bergner, and J. Herz. 2004. Apolipoprotein E receptors are required for reelin-induced proteasomal degradation of the neuronal adaptor protein Disabled-1. J. Biol. Chem. 279:33471-33479. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
