ZPR1 is essential for survival and is required for localization of the survival motor neurons (SMN) protein to Cajal bodies
- PMID: 15767679
- PMCID: PMC1061650
- DOI: 10.1128/MCB.25.7.2744-2756.2005
ZPR1 is essential for survival and is required for localization of the survival motor neurons (SMN) protein to Cajal bodies
Abstract
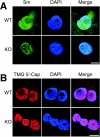
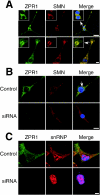
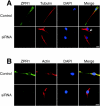
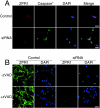
Mutation of the survival motor neurons 1 (SMN1) gene causes motor neuron apoptosis and represents the major cause of spinal muscular atrophy in humans. Biochemical studies have established that the SMN protein plays an important role in spliceosomal small nuclear ribonucleoprotein (snRNP) biogenesis and that the SMN complex can interact with the zinc finger protein ZPR1. Here we report that targeted ablation of the Zpr1 gene in mice disrupts the subcellular localization of both SMN and spliceosomal snRNPs. Specifically, SMN localization to Cajal bodies and gems was not observed in cells derived from Zpr1-/- embryos and the amount of cytoplasmic snRNP detected in Zpr1-/- embryos was reduced compared with that in wild-type embryos. We found that Zpr1-/- mice die during early embryonic development, with reduced proliferation and increased apoptosis. These effects of Zpr1 gene disruption were confirmed and extended in studies of cultured motor neuron-like cells using small interfering RNA-mediated Zpr1 gene suppression; ZPR1 deficiency caused growth cone retraction, axonal defects, and apoptosis. Together, these data indicate that ZPR1 contributes to the regulation of SMN complexes and that it is essential for cell survival.
Figures



Similar articles
-
Distinct domains of the spinal muscular atrophy protein SMN are required for targeting to Cajal bodies in mammalian cells.J Cell Sci. 2006 Feb 15;119(Pt 4):680-92. doi: 10.1242/jcs.02782. Epub 2006 Jan 31. J Cell Sci. 2006. PMID: 16449324
-
The SMN binding protein Gemin2 is not involved in motor axon outgrowth.Dev Neurobiol. 2008 Feb 1;68(2):182-94. doi: 10.1002/dneu.20582. Dev Neurobiol. 2008. PMID: 18000835
-
A role for complexes of survival of motor neurons (SMN) protein with gemins and profilin in neurite-like cytoplasmic extensions of cultured nerve cells.Exp Cell Res. 2005 Sep 10;309(1):185-97. doi: 10.1016/j.yexcr.2005.05.014. Exp Cell Res. 2005. PMID: 15975577
-
The SMN complex.Exp Cell Res. 2004 May 15;296(1):51-6. doi: 10.1016/j.yexcr.2004.03.022. Exp Cell Res. 2004. PMID: 15120993 Review.
-
Pathogenesis of proximal autosomal recessive spinal muscular atrophy.Acta Neuropathol. 2008 Sep;116(3):223-34. doi: 10.1007/s00401-008-0411-1. Epub 2008 Jul 16. Acta Neuropathol. 2008. PMID: 18629520 Review.
Cited by
-
Molecular Mechanisms of Neurodegeneration in Spinal Muscular Atrophy.J Exp Neurosci. 2016 Mar 23;10:39-49. doi: 10.4137/JEN.S33122. eCollection 2016. J Exp Neurosci. 2016. PMID: 27042141 Free PMC article. Review.
-
Zinc finger protein ZPR1: promising survival motor neuron protein-dependent modifier for the rescue of spinal muscular atrophy.Neural Regen Res. 2022 Oct;17(10):2225-2227. doi: 10.4103/1673-5374.335798. Neural Regen Res. 2022. PMID: 35259840 Free PMC article. No abstract available.
-
ZNF259 inhibits non-small cell lung cancer cells proliferation and invasion by FAK-AKT signaling.Cancer Manag Res. 2017 Dec 14;9:879-889. doi: 10.2147/CMAR.S150614. eCollection 2017. Cancer Manag Res. 2017. PMID: 29276408 Free PMC article.
-
Deregulation of ZPR1 causes respiratory failure in spinal muscular atrophy.Sci Rep. 2017 Aug 15;7(1):8295. doi: 10.1038/s41598-017-07603-z. Sci Rep. 2017. PMID: 28811488 Free PMC article.
-
Molecular Factors Involved in Spinal Muscular Atrophy Pathways as Possible Disease-modifying Candidates.Curr Genomics. 2018 Aug;19(5):339-355. doi: 10.2174/1389202919666180101154916. Curr Genomics. 2018. PMID: 30065610 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
