Normal cell cycle and checkpoint responses in mice and cells lacking Cdc25B and Cdc25C protein phosphatases
- PMID: 15767688
- PMCID: PMC1061644
- DOI: 10.1128/MCB.25.7.2853-2860.2005
Normal cell cycle and checkpoint responses in mice and cells lacking Cdc25B and Cdc25C protein phosphatases
Abstract
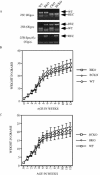
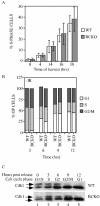
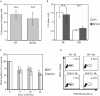
The Cdc25 family of protein phosphatases positively regulates cell division by activating cyclin-dependent protein kinases (CDKs). In humans and rodents, there are three Cdc25 family members--denoted Cdc25A, Cdc25B, and Cdc25C--that can be distinguished based on their subcellular compartmentalizations, their abundances and/or activities throughout the cell cycle, the CDKs that they target for activation, and whether they are overexpressed in human cancers. In addition, murine forms of Cdc25 exhibit distinct patterns of expression throughout development and in adult tissues. These properties suggest that individual Cdc25 family members contribute distinct biological functions in embryonic and adult cell cycles of mammals. Interestingly, mice with Cdc25C disrupted are healthy, and cells derived from these mice exhibit normal cell cycles and checkpoint responses. Cdc25B-/- mice are also generally normal (although females are sterile), and cells derived from Cdc25B-/- mice have normal cell cycles. Here we report that mice lacking both Cdc25B and Cdc25C are obtained at the expected Mendelian ratios, indicating that Cdc25B and Cdc25C are not required for mouse development or mitotic entry. Furthermore, cell cycles, DNA damage responses, and Cdc25A regulation are normal in cells lacking Cdc25B and Cdc25C. These findings indicate that Cdc25A, or possibly other phosphatases, is able to functionally compensate for the loss of Cdc25B and Cdc25C in mice.
Figures

References
-
- Abrieu, A., T. Brassac, S. Galas, D. Fisher, J. C. Labbe, and M. Doree. 1998. The Polo-like kinase Plx1 is a component of the MPF amplification loop at the G2/M-phase transition of the cell cycle in Xenopus eggs. J. Cell Sci. 111:1751-1757. - PubMed
-
- Barlow, C., K. D. Brown, C. X. Deng, D. A. Tagle, and A. Wynshaw-Boris. 1997. Atm selectively regulates distinct p53-dependent cell-cycle checkpoint and apoptotic pathways. Nat. Genet. 17:453-456. - PubMed
-
- Bernardi, R., D. A. Lieberman, and B. Hoffman. 2000. Cdc25A stability is controlled by the ubiquitin-proteasome pathway during cell cycle progression and terminal differentiation. Oncogene 19:2447-2454. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
