The role of endothelial PI3Kgamma activity in neutrophil trafficking
- PMID: 15769890
- PMCID: PMC1895128
- DOI: 10.1182/blood-2005-01-0023
The role of endothelial PI3Kgamma activity in neutrophil trafficking
Abstract
Phosphoinositide 3-kinase gamma (PI3Kgamma) in neutrophils plays a critical role in the directed migration of these cells into inflamed tissues. In this study, we demonstrate the importance of the endothelial component of PI3Kgamma activity relative to its leukocyte counterpart in supporting neutrophil interactions with the inflamed vessel wall. Despite the reconstitution of class-Ib PI3K function in neutrophils of p110gamma-/- mice, we observed a 45% reduction in accumulation of these cells in an acute lung injury model. Mechanistically, this appears to result from a perturbation in selectin-mediated adhesion as manifested by a 70% reduction in wild-type (WT) neutrophil attachment to and 17-fold increase in rolling velocities on p110gamma-/- microvessels in vivo in response to tumor necrosis factor alpha (TNFalpha). This alteration in adhesion was further augmented by a deficiency in p110delta, suggesting that the activity of both catalytic subunits is required for efficient capture of neutrophils by cytokine-stimulated endothelium. Interestingly, E-selectin-mediated adhesion in p110gamma-/-) mice was impaired by more than 95%, but no defect in nuclear factor kappa B (NF-kappaB)-induced gene expression was observed. These findings suggest a previously unrecognized partnership between class-I PI3Ks expressed in leukocytes and endothelium, the combination of which is required for the efficient trafficking of immunocompetent cells to sites of inflammation.
Figures
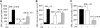
 , p110γ–/– reconstituted with GFP–/+ p110γ+/+ fetal liver cells. (B) ▪ indicates WT littermate (GFP–/+ p110δ+/+); ▪, GFP–/+ p110δ–/–; and
, p110γ–/– reconstituted with GFP–/+ p110γ+/+ fetal liver cells. (B) ▪ indicates WT littermate (GFP–/+ p110δ+/+); ▪, GFP–/+ p110δ–/–; and  , p110δ–/– reconstituted with GFP–/+ p110δ+/+ fetal liver cells. (C) ▪ indicates WT littermate (GFP–/+ p110δ+/+); ▪, WT littermate plus mAB CL37; and
, p110δ–/– reconstituted with GFP–/+ p110δ+/+ fetal liver cells. (C) ▪ indicates WT littermate (GFP–/+ p110δ+/+); ▪, WT littermate plus mAB CL37; and  , WT littermate plus mAb 9A9. Mean values (± SD) are shown for 8 mice in each experimental or control group. *P < .05.
, WT littermate plus mAb 9A9. Mean values (± SD) are shown for 8 mice in each experimental or control group. *P < .05.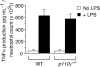
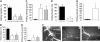
 , p110γ–/– or p110δ–/– reconstituted with WT GFP–/+ fetal liver cells. Results represent the mean plus or minus SD; *P < .05 as compared with WT littermates. n = number of mice/venules analyzed. Rolling velocities for consecutive interacting cells (n = 30 per venule) in mice (C) deficient in or chimeric for p110γ, or (D) deficient in or chimeric for p110δ. Data represent the mean ± SD for more than 150 cells per experimental condition; *P < .05. (E) Rolling fraction and (G) rolling velocities for p110γ–/–/p110δ–/– mice reconstituted with FLC from GFP–/+ WT fetal liver cells (▪). ▪ indicates WT littermates reconstituted with GFP–/+ WT fetal liver cells. *P < .05 as compared with controls. (F) Representative intravital micrographs depicting the extent of neutrophil adhesion to and transmigration across CM venules in WT mice (i) and p110γ–/– (ii) or p110δ–/– (iii) animals reconstituted with WT FLCs (3 hours after stimulation with TNFα).
, p110γ–/– or p110δ–/– reconstituted with WT GFP–/+ fetal liver cells. Results represent the mean plus or minus SD; *P < .05 as compared with WT littermates. n = number of mice/venules analyzed. Rolling velocities for consecutive interacting cells (n = 30 per venule) in mice (C) deficient in or chimeric for p110γ, or (D) deficient in or chimeric for p110δ. Data represent the mean ± SD for more than 150 cells per experimental condition; *P < .05. (E) Rolling fraction and (G) rolling velocities for p110γ–/–/p110δ–/– mice reconstituted with FLC from GFP–/+ WT fetal liver cells (▪). ▪ indicates WT littermates reconstituted with GFP–/+ WT fetal liver cells. *P < .05 as compared with controls. (F) Representative intravital micrographs depicting the extent of neutrophil adhesion to and transmigration across CM venules in WT mice (i) and p110γ–/– (ii) or p110δ–/– (iii) animals reconstituted with WT FLCs (3 hours after stimulation with TNFα).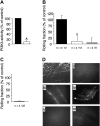
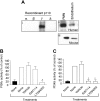
 ) 1 hour prior to induction of inflammation with TNFα or (C) in those that also lacked p110γ. Data represent the mean plus or minus SD and are normalized as a percentage of control; *P < .05 as compared with control. n = number of mice /venules analyzed. (D) In vivo imaging of E-selectin expression on CM venules in mice with or without prior stimulation with TNFα. Fluorescent Qdots coated with an antibody that recognizes murine E-selectin were injected intravenously into animals and immunolocalization of this adhesion molecule was visualized by intravital microscopy. Transmitted light (i) and epifluorescence (ii) images depict staining of venules in P-selectin–/– mice in the absence of TNFα stimulation. Representative micrographs of inflamed venules in P-selectin–/– animals pretreated with (iii) IC87114 or (iv) lacking p110γ, respectively, or mice deficient in E-selectin in the absence (v) or presence (vi) of IC87114.
) 1 hour prior to induction of inflammation with TNFα or (C) in those that also lacked p110γ. Data represent the mean plus or minus SD and are normalized as a percentage of control; *P < .05 as compared with control. n = number of mice /venules analyzed. (D) In vivo imaging of E-selectin expression on CM venules in mice with or without prior stimulation with TNFα. Fluorescent Qdots coated with an antibody that recognizes murine E-selectin were injected intravenously into animals and immunolocalization of this adhesion molecule was visualized by intravital microscopy. Transmitted light (i) and epifluorescence (ii) images depict staining of venules in P-selectin–/– mice in the absence of TNFα stimulation. Representative micrographs of inflamed venules in P-selectin–/– animals pretreated with (iii) IC87114 or (iv) lacking p110γ, respectively, or mice deficient in E-selectin in the absence (v) or presence (vi) of IC87114.
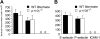
References
-
- Fruman DA, Cantley LC. Phosphoinositide 3-kinase in immunological systems. Semin Immunol. 2002;14: 7-18. - PubMed
-
- Wymann MP, Sozzani S, Altruda F, Mantovani A, Hirsch E. Lipids on the move: phosphoinositide 3-kinases in leukocyte function. Immunol Today. 2000;21: 260-264. - PubMed
-
- Koyasu S. The role of PI3K in immune cells. Nat Immunol. 2003;4: 313-319. - PubMed
-
- Okkenhaug K, Vanhaesebroeck B. PI3K in lymphocyte development, differentiation and activation. Nat Rev Immunol. 2003;3: 317-330. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

