Induction of tolerance to factor VIII inhibitors by gene therapy with immunodominant A2 and C2 domains presented by B cells as Ig fusion proteins
- PMID: 15769892
- PMCID: PMC1894991
- DOI: 10.1182/blood-2004-11-4274
Induction of tolerance to factor VIII inhibitors by gene therapy with immunodominant A2 and C2 domains presented by B cells as Ig fusion proteins
Abstract
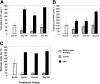
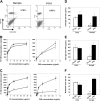
Up to 30% of patients with hemophilia A given therapeutic factor VIII (fVIII) can make inhibitory antibodies, the majority of which are reactive with its C2 and A2 domains. We have previously demonstrated that antigen-specific tolerance to several antigens can be induced by lipopolysaccharide (LPS)-activated B-cell blasts transduced with immunoglobulin (IgG)-antigen fusion constructs. To apply this system to hemophilia A inhibitor formation, we created retroviral vectors expressing fVIII amino acids S2173-Y2332 (C2 domain) and S373-R740 (A2 domain) in frame with an IgG heavy chain backbone. These vectors were transduced into B-cell blasts to induce tolerance in both naive and fVIII-primed hemophilic (E16 fVIII(-/-)) mice. Thus, treatment of E16 fVIII(-/-) mice with B cells expressing fVIII C2 and A2 domains led to tolerance in terms of specific humoral response (including inhibitory antibody titers) and cellular responses to fVIII and its C2 or A2 domains. Moreover, a significant reduction in immune responses to fVIII could be achieved in immunized hemophilic mice with existing anti-fVIII titers. This hyporesponsive state persisted for at least 2 months and withstood additional challenge with fVIII. Further experiments, in which mice were treated with a depleting monoclonal anti-CD25, suggested that a regulatory T cell may be required for the tolerogenic effect of transduced B cells. These findings demonstrate that B-cell presentation of fVIII domains on an Ig backbone specifically prevents or decreases existing antibodies in hemophilia A mice.
Figures



Similar articles
-
Immune tolerance induced by platelet-targeted factor VIII gene therapy in hemophilia A mice is CD4 T cell mediated.J Thromb Haemost. 2017 Oct;15(10):1994-2004. doi: 10.1111/jth.13800. Epub 2017 Sep 11. J Thromb Haemost. 2017. PMID: 28799202 Free PMC article.
-
Targeting Antigen-Specific B Cells Using Antigen-Expressing Transduced Regulatory T Cells.J Immunol. 2018 Sep 1;201(5):1434-1441. doi: 10.4049/jimmunol.1701800. Epub 2018 Jul 18. J Immunol. 2018. PMID: 30021767 Free PMC article.
-
Persistent splenic-derived IgMs preferentially recognize factor VIII A2 and C2 domain epitopes but do not alter antibody production.J Thromb Haemost. 2025 Feb;23(2):440-457. doi: 10.1016/j.jtha.2024.10.017. Epub 2024 Oct 28. J Thromb Haemost. 2025. PMID: 39476969
-
Inhibitors in hemophilia A: mechanisms of inhibition, management and perspectives.Blood Coagul Fibrinolysis. 2004 Mar;15(2):109-24. doi: 10.1097/00001721-200403000-00001. Blood Coagul Fibrinolysis. 2004. PMID: 15090997 Review.
-
The molecular mechanisms of immunomodulation and tolerance induction to factor VIII.J Thromb Haemost. 2009 Sep;7(9):1446-56. doi: 10.1111/j.1538-7836.2009.03538.x. Epub 2009 Jul 6. J Thromb Haemost. 2009. PMID: 19583822 Review.
Cited by
-
Transient blockade of the inducible costimulator pathway generates long-term tolerance to factor VIII after nonviral gene transfer into hemophilia A mice.Blood. 2008 Sep 1;112(5):1662-72. doi: 10.1182/blood-2008-01-128413. Epub 2008 Jun 23. Blood. 2008. PMID: 18574023 Free PMC article.
-
CD8+ regulatory T cells are responsible for GAD-IgG gene-transferred tolerance induction in NOD mice.Immunology. 2009 Jan;126(1):123-31. doi: 10.1111/j.1365-2567.2008.02884.x. Epub 2008 Jun 20. Immunology. 2009. PMID: 18624731 Free PMC article.
-
Recombinant factor VIII Fc (rFVIIIFc) fusion protein reduces immunogenicity and induces tolerance in hemophilia A mice.Cell Immunol. 2016 Mar;301:30-9. doi: 10.1016/j.cellimm.2015.12.008. Epub 2015 Dec 29. Cell Immunol. 2016. PMID: 26775174 Free PMC article.
-
Factor VIII delivered by haematopoietic stem cell-derived B cells corrects the phenotype of haemophilia A mice.Thromb Haemost. 2011 Apr;105(4):676-87. doi: 10.1160/TH10-11-0725. Epub 2011 Jan 25. Thromb Haemost. 2011. PMID: 21264447 Free PMC article.
-
Prolonged activity of a recombinant factor VIII-Fc fusion protein in hemophilia A mice and dogs.Blood. 2012 Mar 29;119(13):3024-30. doi: 10.1182/blood-2011-08-367813. Epub 2012 Jan 13. Blood. 2012. PMID: 22246033 Free PMC article.
References
-
- Jacquemin MG, Saint-Remy JM. FVIII alloantibodies in hemophilia. Curr Opin Hematol. 2004;11: 146-150. - PubMed
-
- Villard S, Lacroix-Desmazes S, Kieber-Emmons T, et al. Peptide decoys selected by phage display block in vitro and in vivo activity of a human anti-fVIII inhibitor. Blood. 2003;102: 949-952. - PubMed
-
- Barrow RT, Healey JF, Gailani D, Scandella D, Lollar P. Reduction of the antigenicity of fVIII toward complex inhibitory antibody plasmas using multiply-substituted hybrid human/porcine fVIII molecules. Blood. 2000;15; 95:564-568. - PubMed
-
- Ananyeva NM, Lacroix-Desmazes S, Hauser CA, et al. Inhibitors in hemophilia A: mechanisms of inhibition, management and perspectives. Blood Coagul Fibrinolysis. 2004;15: 109-124. - PubMed
-
- Qian J, Collins M, Sharpe AH, Hoyer LW. Prevention and treatment of factor VIII inhibitors in murine hemophilia A. Blood. 2000;95: 1324-1329. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

