(N)-methanocarba 2,N6-disubstituted adenine nucleosides as highly potent and selective A3 adenosine receptor agonists
- PMID: 15771421
- PMCID: PMC3463111
- DOI: 10.1021/jm049580r
(N)-methanocarba 2,N6-disubstituted adenine nucleosides as highly potent and selective A3 adenosine receptor agonists
Abstract
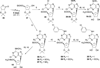
A series of ring-constrained (N)-methanocarba-5'-uronamide 2,N(6)-disubstituted adenine nucleosides have been synthesized via Mitsunobu condensation of the nucleobase precursor with a pseudosugar ring containing a 5'-ester functionality. Following appropriate functionalization of the adenine ring, the ester group was converted to the 5'-N-methylamide. The compounds, mainly 2-chloro-substituted derivatives, were tested in both binding and functional assays at human adenosine receptors (ARs), and many were found to be highly potent and selective A(3)AR agonists. Selected compounds were compared in binding to the rat A(3)AR to assess their viability for testing in rat disease models. The N(6)-(3-chlorobenzyl) and N(6)-(3-bromobenzyl) analogues displayed K(i) values at the human A(3)AR of 0.29 and 0.38 nM, respectively. Other subnanomolar affinities were observed for the following N(6) derivatives: 2,5-dichlorobenzyl, 5-iodo-2-methoxybenzyl, trans-2-phenyl-1-cyclopropyl, and 2,2-diphenylethyl. Selectivity for the human A(3)AR in comparison to the A(1)AR was the following (fold): the N(6)-(2,2-diphenylethyl) analogue 34 (1900), the N(6)-(2,5-dimethoxybenzyl) analogue 26 (1200), the N(6)-(2,5-dichlorobenzyl) and N(6)-(2-phenyl-1-cyclopropyl) analogues 20 and 33 (1000), and the N(6)-(3-substituted benzyl) analogues 17, 18, 28, and 29 (700-900). Typically, even greater selectivity ratios were obtained in comparison with the A(2A) and A(2B)ARs. The (N)-methanocarba-5'-uronamide analogues were full agonists at the A(3)AR, as indicated by the inhibition of forskolin-stimluated adenylate cyclase at a concentration of 10 microM. The N(6)-(2,2-diphenylethyl) derivative was an A(3)AR agonist in the (N)-methanocarba-5'-uronamide series, although it was an antagonist in the ribose series. Thus, many of the previously known groups that enhance A(3)AR affinity in the 9-riboside series, including those that reduce intrinsic efficacy, may be adapted to the (N)-methanocarba nucleoside series of full agonists.
Figures


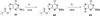

References
-
- Yao L, Burbiel JC, Maass A, Müller CE. Adenosine receptor agonists: from basic medicinal chemistry to clinical development. Expert Opin. Emerging Drugs. 2003;8:537–576. - PubMed
-
- Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414:916–920. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Research Materials

