Disruption of the MAP1B-related protein FUTSCH leads to changes in the neuronal cytoskeleton, axonal transport defects, and progressive neurodegeneration in Drosophila
- PMID: 15772149
- PMCID: PMC1087247
- DOI: 10.1091/mbc.e04-11-1004
Disruption of the MAP1B-related protein FUTSCH leads to changes in the neuronal cytoskeleton, axonal transport defects, and progressive neurodegeneration in Drosophila
Abstract
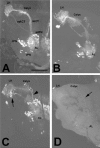
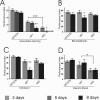
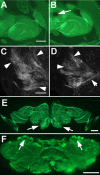
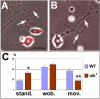
The elaboration of neuronal axons and dendrites is dependent on a functional cytoskeleton. Cytoskeletal components have been shown to play a major role in the maintenance of the nervous system through adulthood, and changes in neurofilaments and microtubule-associated proteins (MAPs) have been linked to a variety of neurodegenerative diseases. Here we show that Futsch, the fly homolog of MAP1B, is involved in progressive neurodegeneration. Although Futsch is widely expressed throughout the CNS, degeneration in futsch(olk) primarily occurs in the olfactory system and mushroom bodies. Consistent with the predicted function of Futsch, we find abnormalities in the microtubule network and defects in axonal transport. Degeneration in the adult brain is preceded by learning deficits, revealing a neuronal dysfunction before detectable levels of cell death. Futsch is negatively regulated by the Drosophila Fragile X mental retardation gene, and a mutation in this gene delays the onset of neurodegeneration in futsch(olk). A similar effect is obtained by expression of either fly or bovine tau, suggesting a certain degree of functional redundancy of MAPs. The futsch(olk) mutants exhibit several characteristics of human neurodegenerative diseases, providing an opportunity to study the role of MAPs in progressive neurodegeneration within an experimentally accessible, in vivo model system.
Figures



References
-
- Blest, A. D. (1961). Some modifications of Holme's silver method for insect central nervous systems. Q. J. Microsc. Sci. 102, 413.
-
- Brandt, R. (2001). Cytoskeletal mechanisms of neuronal degeneration. Cell Tissue Res. 305, 255-265. - PubMed
-
- Buchner, S., Buchner, E., and Hofbauer, A. (1989). Immunohistochemistry of the brain. In: Drosophila: A Laboratory Manual. ed. M. Ashburner, Cold Spring Harbor, NY: Cold Spring Harbor Press, 271-273.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

