The unique N-terminus of R-ras is required for Rac activation and precise regulation of cell migration
- PMID: 15772154
- PMCID: PMC1087249
- DOI: 10.1091/mbc.e03-12-0917
The unique N-terminus of R-ras is required for Rac activation and precise regulation of cell migration
Abstract
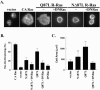
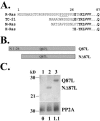
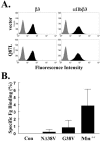
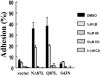
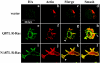
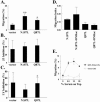
The Ras family GTPase, R-Ras, elicits important integrin-dependent cellular behaviors such as adhesion, spreading and migration. While oncogenic Ras GTPases and R-Ras share extensive sequence homology, R-Ras induces a distinct set of cellular behaviors. To explore the structural basis for these differences, we asked whether the unique N-terminal 26 amino acid extension of R-Ras was responsible for R-Ras-specific signaling events. Using a 32D mouse myeloid cell line, we show that full-length R-Ras activates Rac and induces Rac-dependent cell spreading. In contrast, truncated R-Ras lacking its first 26 amino acids fails to activate Rac, resulting in reduced cell spreading. Truncated R-Ras also stimulates more beta3 integrin-dependent cell migration than full-length R-Ras, suggesting that the N-terminus may negatively regulate cell movement. However, neither the subcellular localization of R-Ras nor its effects on cell adhesion are affected by the presence or absence of the N-terminus. These results indicate that the N-terminus of R-Ras positively regulates specific R-Ras functions such as Rac activation and cell spreading but negatively regulates R-Ras-mediated cell migration.
Figures


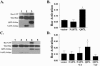

Similar articles
-
RLIP76 (RalBP1) is an R-Ras effector that mediates adhesion-dependent Rac activation and cell migration.J Cell Biol. 2006 Sep 11;174(6):877-88. doi: 10.1083/jcb.200603111. J Cell Biol. 2006. PMID: 16966426 Free PMC article.
-
R-Ras controls membrane protrusion and cell migration through the spatial regulation of Rac and Rho.Mol Biol Cell. 2005 Jan;16(1):84-96. doi: 10.1091/mbc.e04-04-0277. Epub 2004 Nov 3. Mol Biol Cell. 2005. PMID: 15525681 Free PMC article.
-
Activated R-ras, Rac1, PI 3-kinase and PKCepsilon can each restore cell spreading inhibited by isolated integrin beta1 cytoplasmic domains.J Cell Biol. 2000 Dec 25;151(7):1549-60. doi: 10.1083/jcb.151.7.1549. J Cell Biol. 2000. PMID: 11134082 Free PMC article.
-
PI3K and RAC signalling in leukocyte and cancer cell migration.Bull Cancer. 2006 May;93(5):E44-52. Bull Cancer. 2006. PMID: 16777617 Review.
-
Rho family proteins and Ras transformation: the RHOad less traveled gets congested.Oncogene. 1998 Sep 17;17(11 Reviews):1415-38. doi: 10.1038/sj.onc.1202181. Oncogene. 1998. PMID: 9779988 Review.
Cited by
-
Regulation of H-Ras-driven MAPK signaling, transformation and tumorigenesis, but not PI3K signaling and tumor progression, by plasma membrane microdomains.Oncogenesis. 2016 May 30;5(5):e228. doi: 10.1038/oncsis.2016.36. Oncogenesis. 2016. PMID: 27239960 Free PMC article.
-
Integrin activation by Fam38A uses a novel mechanism of R-Ras targeting to the endoplasmic reticulum.J Cell Sci. 2010 Jan 1;123(Pt 1):51-61. doi: 10.1242/jcs.056424. J Cell Sci. 2010. PMID: 20016066 Free PMC article.
-
C3G overexpression in glomerular epithelial cells during anti-GBM-induced glomerulonephritis.Kidney Int. 2009 Jan;75(1):31-40. doi: 10.1038/ki.2008.448. Epub 2008 Sep 10. Kidney Int. 2009. PMID: 18784646 Free PMC article.
-
R-Ras promotes metastasis of cervical cancer epithelial cells.Cancer Immunol Immunother. 2007 Apr;56(4):535-44. doi: 10.1007/s00262-006-0205-z. Epub 2006 Jul 22. Cancer Immunol Immunother. 2007. PMID: 16862428 Free PMC article.
-
The R-Ras/RIN2/Rab5 complex controls endothelial cell adhesion and morphogenesis via active integrin endocytosis and Rac signaling.Cell Res. 2012 Oct;22(10):1479-501. doi: 10.1038/cr.2012.110. Epub 2012 Jul 24. Cell Res. 2012. PMID: 22825554 Free PMC article.
References
-
- Borisy, G. G., and Svitkina, T. M. (2000). Actin machinery: pushing the envelope. Curr. Opin. Cell Biol. 12, 104-112. - PubMed
-
- Calderwood, D. A. (2004). Integrin activation. J. Cell Sci. 117, 657-666. - PubMed
-
- Caron, E., Self, A. J., and Hall, A. (2000). The GTPase Rap1 controls functional activation of macrophage integrin alphaMbeta2 by LPS and other inflammatory mediators. Curr. Biol. 10, 974-978. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

