Low O2 tensions and the prevention of differentiation of hES cells
- PMID: 15772165
- PMCID: PMC554750
- DOI: 10.1073/pnas.0501283102
Low O2 tensions and the prevention of differentiation of hES cells
Abstract
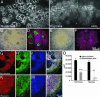
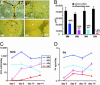
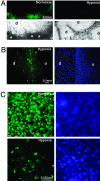
Early-stage mammalian embryos develop in a low O(2) environment (hypoxia). hES cells, however, are generally cultured under an atmosphere of 21% O(2) (normoxia), under which conditions they tend to differentiate spontaneously. Such conditions may not be the most suitable, therefore, for hES cell propagation. Here we have tested two hypotheses. The first hypothesis was that hES cells would grow as well under hypoxic as under normoxic conditions. The second hypothesis was that hypoxic culture would reduce the amount of spontaneous cell differentiation that occurs in hES colonies. Both hypotheses proved to be correct. Cells proliferated as well under 3% and 5% O(2) as they did under 21% O(2), and growth was only slightly reduced at 1% O(2). The appearance of differentiated regions as assessed morphologically, biochemically (by the production of human chorionic gonadotropin and progesterone), and immunohistochemically (by the loss of stage-specific embryonic antigen-4 and Oct-4 and gain of stage-specific embryonic antigen-1 marker expression) was markedly reduced under hypoxic conditions. In addition, hES cell growth under hypoxia provided enhanced formation of embryoid bodies. Hypoxic culture would appear to be necessary to maintain full pluripotency of hES cells.
Figures



References
-
- Thomson, J. A., Itskovitz-Eldor, J., Shapiro, S. S., Waknitz, M. A., Swiergiel, J. J., Marshall, V. S. & Jones, J. M. (1998) Science 282, 1145-1147. - PubMed
-
- Thomson, J. A. & Marshall, V. S. (1998) Curr. Top. Dev. Biol. 38, 133-165. - PubMed
-
- Reubinoff, B. E., Pera, M. F., Fong, C. Y., Trounson, A. & Bongso, A. (2000) Nat. Biotechnol. 18, 399-404. - PubMed
-
- Williams, R., Hilton, D., Pease, S., Willson, T., Stewart, C., Gearing, D., Wagner, E., Metcalf, D., Nicola, N. & Gough, N. (1988) Nature 336, 684-687. - PubMed
-
- Niwa, H. (2001) Cell Struct. Funct. 26, 137-148. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

