T cell receptor binding kinetics required for T cell activation depend on the density of cognate ligand on the antigen-presenting cell
- PMID: 15772168
- PMCID: PMC555720
- DOI: 10.1073/pnas.0500922102
T cell receptor binding kinetics required for T cell activation depend on the density of cognate ligand on the antigen-presenting cell
Abstract
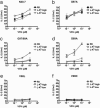
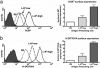
CD8(+) T cells recognize peptides of eight to nine amino acid residues long in the context of MHC class I molecules on the surface of antigen-presenting cells (APCs). This recognition event is highly sensitive, as evidenced by the fact that T cells can be activated by cognate peptide/MHC complex (pMHC) at extremely low densities (1-50 molecules). High sensitivity is particularly valuable for detection of antigens at low density, such as those derived from tumor cells and intracellular pathogens, which can down-modulate cognate pMHCs from the surface of APCs to evade recognition by the adaptive immune system. T cell activation is only triggered in response to interactions between the T cell receptor (TCR) and the pMHC ligand that reach a specific half-life threshold. However, interactions with excessively long half-lives result in impaired T cell activation. Thus, efficient T cell activation by pMHC on the surface of APCs requires an optimal dwell time of TCR-pMHC interaction. Here, we show that, although this is a requirement at low cognate pMHC density on the APC surface, at high epitope density there is no impairment of T cell activation by extended TCR-pMHC dwell times. This observation was predicted by mathematical simulations for T cell activation by pMHC at different densities and supported by experiments performed on APCs selected for varied expression of cognate pMHC. According to these results, effective T cell activation depends on a complex interplay between inherent TCR-pMHC binding kinetics and the epitope density on the APC.
Figures




Similar articles
-
Modulation of T cell function by TCR/pMHC binding kinetics.Immunobiology. 2006;211(1-2):47-64. doi: 10.1016/j.imbio.2005.09.003. Epub 2006 Jan 4. Immunobiology. 2006. PMID: 16446170 Review.
-
T-cell receptor triggering is critically dependent on the dimensions of its peptide-MHC ligand.Nature. 2005 Jul 28;436(7050):578-82. doi: 10.1038/nature03843. Nature. 2005. PMID: 16049493
-
The duration of TCR/pMHC interactions regulates CTL effector function and tumor-killing capacity.Eur J Immunol. 2009 Aug;39(8):2259-69. doi: 10.1002/eji.200939341. Eur J Immunol. 2009. PMID: 19637198
-
Efficient T cell activation requires an optimal dwell-time of interaction between the TCR and the pMHC complex.Nat Immunol. 2001 Mar;2(3):229-34. doi: 10.1038/85286. Nat Immunol. 2001. PMID: 11224522
-
Spatial coordination of CD8 and TCR molecules controls antigen recognition by CD8+ T-cells.FEBS Lett. 2005 Jun 13;579(15):3336-41. doi: 10.1016/j.febslet.2005.04.025. Epub 2005 Apr 26. FEBS Lett. 2005. PMID: 15913613 Review.
Cited by
-
Coreceptor CD8-driven modulation of T cell antigen receptor specificity.J Theor Biol. 2007 Nov 21;249(2):395-408. doi: 10.1016/j.jtbi.2007.08.002. Epub 2007 Aug 8. J Theor Biol. 2007. PMID: 17869274 Free PMC article.
-
Defining and Studying B Cell Receptor and TCR Interactions.J Immunol. 2023 Aug 1;211(3):311-322. doi: 10.4049/jimmunol.2300136. J Immunol. 2023. PMID: 37459189 Free PMC article.
-
Biomaterial Strategies for Immunomodulation.Annu Rev Biomed Eng. 2015;17:317-49. doi: 10.1146/annurev-bioeng-071813-104814. Epub 2015 Sep 29. Annu Rev Biomed Eng. 2015. PMID: 26421896 Free PMC article. Review.
-
Impact of cancer evolution on immune surveillance and checkpoint inhibitor response.Semin Cancer Biol. 2022 Sep;84:89-102. doi: 10.1016/j.semcancer.2021.02.013. Epub 2021 Feb 22. Semin Cancer Biol. 2022. PMID: 33631295 Free PMC article. Review.
-
Chemical Modification of Influenza CD8+ T-Cell Epitopes Enhances Their Immunogenicity Regardless of Immunodominance.PLoS One. 2016 Jun 22;11(6):e0156462. doi: 10.1371/journal.pone.0156462. eCollection 2016. PLoS One. 2016. PMID: 27333291 Free PMC article.
References
-
- Kalergis, A. M. (2003) Curr. Pharm. Des. 9, 233-244. - PubMed
-
- Garcia, K. C., Teyton, L. & Wilson, I. A. (1999) Annu. Rev. Immunol. 17, 369-397. - PubMed
-
- Kalergis, A. M. & Nathenson, S. G. (2000) J. Immunol. 165, 280-285. - PubMed
-
- Eisen, H. N., Sykulev, Y. & Tsomides, T. J. (1996) Adv. Protein Chem. 49, 1-56. - PubMed
-
- Sykulev, Y., Joo, M., Vturina, I., Tsomides, T. J. & Eisen, H. N. (1996) Immunity 4, 565-571. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

