Human alpha-defensins neutralize anthrax lethal toxin and protect against its fatal consequences
- PMID: 15772169
- PMCID: PMC555714
- DOI: 10.1073/pnas.0500508102
Human alpha-defensins neutralize anthrax lethal toxin and protect against its fatal consequences
Abstract
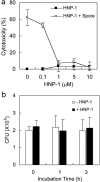
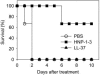
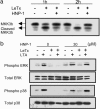
Anthrax caused by Bacillus anthracis represents a major bioterroristic threat. B. anthracis produces lethal toxin (LeTx), a combination of lethal factor (LF) and protective antigen that plays a major role in anthrax pathogenesis. We demonstrate that human neutrophil alpha-defensins are potent inhibitors of LF. The inhibition of LF by human neutrophil protein (HNP-1) was noncompetitive. HNP-1 inhibited cleavage of a mitogen-activated protein kinase kinase and restored impaired mitogen-activated protein kinase signaling in LeTx-treated macrophages. HNP-1 rescued murine macrophages from B. anthracis-induced cytotoxicity, and in vivo treatment with HNP-1-3 protected mice against the fatal consequences of LeTx.
Figures



Similar articles
-
Human alpha-defensins inhibit Clostridium difficile toxin B.Gastroenterology. 2008 Jun;134(7):2049-58. doi: 10.1053/j.gastro.2008.03.008. Epub 2008 Mar 10. Gastroenterology. 2008. PMID: 18435932
-
Cisplatin inhibition of anthrax lethal toxin.Antimicrob Agents Chemother. 2006 Aug;50(8):2658-65. doi: 10.1128/AAC.01412-05. Antimicrob Agents Chemother. 2006. PMID: 16870755 Free PMC article.
-
Proteomic analyses of murine macrophages treated with Bacillus anthracis lethal toxin.Microb Pathog. 2006 Oct-Nov;41(4-5):157-67. doi: 10.1016/j.micpath.2006.07.002. Epub 2006 Sep 1. Microb Pathog. 2006. PMID: 16950595
-
Retrocyclins kill bacilli and germinating spores of Bacillus anthracis and inactivate anthrax lethal toxin.J Biol Chem. 2006 Oct 27;281(43):32755-64. doi: 10.1074/jbc.M603614200. Epub 2006 Jun 21. J Biol Chem. 2006. PMID: 16790431 Free PMC article.
-
Potent inhibition of tumor angiogenesis by the matrix metalloproteinase-activated anthrax lethal toxin: implications for broad anti-tumor efficacy.Cell Cycle. 2008 Mar 15;7(6):745-9. doi: 10.4161/cc.7.6.5627. Epub 2008 Jan 18. Cell Cycle. 2008. PMID: 18245947 Review.
Cited by
-
Human monoclonal antibodies against anthrax lethal factor and protective antigen act independently to protect against Bacillus anthracis infection and enhance endogenous immunity to anthrax.Infect Immun. 2007 Nov;75(11):5425-33. doi: 10.1128/IAI.00261-07. Epub 2007 Jul 23. Infect Immun. 2007. PMID: 17646360 Free PMC article.
-
Capsular antigen fraction 1 and Pla modulate the susceptibility of Yersinia pestis to pulmonary antimicrobial peptides such as cathelicidin.Infect Immun. 2008 Apr;76(4):1456-64. doi: 10.1128/IAI.01197-07. Epub 2008 Jan 28. Infect Immun. 2008. PMID: 18227173 Free PMC article.
-
Molecular determinants for a cardiovascular collapse in anthrax.Front Biosci (Elite Ed). 2014 Jan 1;6(1):139-47. doi: 10.2741/e697. Front Biosci (Elite Ed). 2014. PMID: 24389148 Free PMC article. Review.
-
Sometimes it takes two to tango: contributions of dimerization to functions of human α-defensin HNP1 peptide.J Biol Chem. 2012 Mar 16;287(12):8944-53. doi: 10.1074/jbc.M111.332205. Epub 2012 Jan 23. J Biol Chem. 2012. PMID: 22270360 Free PMC article.
-
Cooperative Function of LL-37 and HNP1 Protects Mammalian Cell Membranes from Lysis.Biophys J. 2020 Dec 15;119(12):2440-2450. doi: 10.1016/j.bpj.2020.10.031. Epub 2020 Nov 4. Biophys J. 2020. PMID: 33157121 Free PMC article.
References
-
- Turnbull, P. C. (1999) J. Appl. Microbiol. 87, 237-240. - PubMed
-
- Duesbery, N. S., Webb, C. P., Leppla, S. H., Gordon, V. M., Klimpel, K. R., Copeland, T. D., Ahn, N. G., Oskarsson, M. K., Fukasawa, K., Paull, K. D. & Vande Woude, G. F. (1998) Science 280, 734-737. - PubMed
-
- Bradley, K. A., Mogridge, J., Mourez, M., Collier, R. J. & Young, J. A. (2001) Nature 414, 225-229. - PubMed
-
- Collier, R. J. & Young, J. A. (2003) Annu. Rev. Cell Dev. Biol. 19, 45-70. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

