Sites and regulation of auxin biosynthesis in Arabidopsis roots
- PMID: 15772288
- PMCID: PMC1087988
- DOI: 10.1105/tpc.104.029272
Sites and regulation of auxin biosynthesis in Arabidopsis roots
Abstract
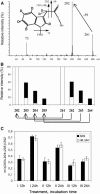
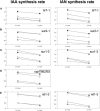
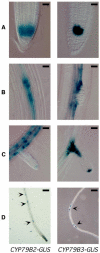
Auxin has been shown to be important for many aspects of root development, including initiation and emergence of lateral roots, patterning of the root apical meristem, gravitropism, and root elongation. Auxin biosynthesis occurs in both aerial portions of the plant and in roots; thus, the auxin required for root development could come from either source, or both. To monitor putative internal sites of auxin synthesis in the root, a method for measuring indole-3-acetic acid (IAA) biosynthesis with tissue resolution was developed. We monitored IAA synthesis in 0.5- to 2-mm sections of Arabidopsis thaliana roots and were able to identify an important auxin source in the meristematic region of the primary root tip as well as in the tips of emerged lateral roots. Lower but significant synthesis capacity was observed in tissues upward from the tip, showing that the root contains multiple auxin sources. Root-localized IAA synthesis was diminished in a cyp79B2 cyp79B3 double knockout, suggesting an important role for Trp-dependent IAA synthesis pathways in the root. We present a model for how the primary root is supplied with auxin during early seedling development.
Figures



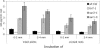
References
-
- Åstot, C., Dolezal, K., Moritz, T., and Sandberg, G. (2000). Deuterium in vivo labelling of cytokinins in Arabidopsis thaliana analysed by capillary liquid chromatography/frit-fast atom bombardment mass spectrometry. J. Mass Spectrom. 35, 13–22. - PubMed
-
- Bartel, B., LeClere, S., Magidin, M., and Zolman, B.K. (2001). Inputs to the active indole-3-acetic acid pool: De novo synthesis, conjugate hydrolysis, and indole-3-butyric acid β-oxidation. J. Plant Growth Regul. 20, 198–216.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

