Leucine-rich repeat receptor-like kinase1 is a key membrane-bound regulator of abscisic acid early signaling in Arabidopsis
- PMID: 15772289
- PMCID: PMC1087989
- DOI: 10.1105/tpc.104.027474
Leucine-rich repeat receptor-like kinase1 is a key membrane-bound regulator of abscisic acid early signaling in Arabidopsis
Abstract
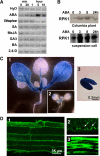
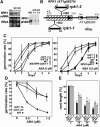
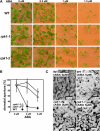
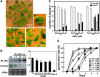
Abscisic acid (ABA) is important in seed maturation, seed dormancy, stomatal closure, and stress response. Many genes that function in ABA signal transduction pathways have been identified. However, most important signaling molecules involved in the perception of the ABA signal or with ABA receptors have not been identified yet. Receptor-like kinase1 (RPK1), a Leu-rich repeat (LRR) receptor kinase in the plasma membrane, is upregulated by ABA in Arabidopsis thaliana. Here, we show the phenotypes of T-DNA insertion mutants and RPK1-antisense plants. Repression of RPK1 expression in Arabidopsis decreased sensitivity to ABA during germination, growth, and stomatal closure; microarray and RNA gel analysis showed that many ABA-inducible genes are downregulated in these plants. Furthermore, overexpression of the RPK1 LRR domain alone or fused with the Brassinosteroid-insensitive1 kinase domain in plants resulted in phenotypes indicating ABA sensitivity. RPK1 is involved in the main ABA signaling pathway and in early ABA perception in Arabidopsis.
Figures



Similar articles
-
Age-dependent action of an ABA-inducible receptor kinase, RPK1, as a positive regulator of senescence in Arabidopsis leaves.Plant Cell Physiol. 2011 Apr;52(4):651-62. doi: 10.1093/pcp/pcr026. Epub 2011 Mar 7. Plant Cell Physiol. 2011. PMID: 21382977
-
Arabidopsis thaliana RECEPTOR DEAD KINASE1 Functions as a Positive Regulator in Plant Responses to ABA.Mol Plant. 2017 Feb 13;10(2):223-243. doi: 10.1016/j.molp.2016.11.011. Epub 2016 Dec 3. Mol Plant. 2017. PMID: 27923613
-
The Arabidopsis Ca(2+) -dependent protein kinase CPK12 negatively regulates abscisic acid signaling in seed germination and post-germination growth.New Phytol. 2011 Oct;192(1):61-73. doi: 10.1111/j.1469-8137.2011.03793.x. Epub 2011 Jun 21. New Phytol. 2011. PMID: 21692804
-
Abscisic acid signaling.Curr Opin Cell Biol. 1995 Apr;7(2):232-8. doi: 10.1016/0955-0674(95)80033-6. Curr Opin Cell Biol. 1995. PMID: 7612276 Review.
-
ABA transport and transporters.Trends Plant Sci. 2013 Jun;18(6):325-33. doi: 10.1016/j.tplants.2013.01.007. Epub 2013 Mar 1. Trends Plant Sci. 2013. PMID: 23453706 Review.
Cited by
-
Genomic investigation of duplication, functional conservation, and divergence in the LRR-RLK Family of Saccharum.BMC Genomics. 2024 Feb 9;25(1):165. doi: 10.1186/s12864-024-10073-z. BMC Genomics. 2024. PMID: 38336615 Free PMC article.
-
Diversity, classification and function of the plant protein kinase superfamily.Philos Trans R Soc Lond B Biol Sci. 2012 Sep 19;367(1602):2619-39. doi: 10.1098/rstb.2012.0003. Philos Trans R Soc Lond B Biol Sci. 2012. PMID: 22889912 Free PMC article.
-
Hope for Humpty Dumpty: systems biology of cellular signaling.Plant Physiol. 2010 Feb;152(2):470-9. doi: 10.1104/pp.109.151266. Epub 2009 Dec 23. Plant Physiol. 2010. PMID: 20032076 Free PMC article. No abstract available.
-
Studies of abscisic acid perception finally flower.Plant Cell. 2006 Apr;18(4):786-91. doi: 10.1105/tpc.106.041129. Plant Cell. 2006. PMID: 16595396 Free PMC article. Review. No abstract available.
-
Characterization and expression analysis of SOMATIC EMBRYOGENESIS RECEPTOR KINASE (SERK) genes in sexual and apomictic Paspalum notatum.Plant Mol Biol. 2014 Mar;84(4-5):479-95. doi: 10.1007/s11103-013-0146-9. Epub 2013 Oct 22. Plant Mol Biol. 2014. PMID: 24146222
References
-
- Clark, S.E., Williams, R.W., and Meyerowitz, E.M. (1997). The CLAVATA1 gene encodes a putative receptor kinase that controls shoot and floral meristem size in Arabidopsis. Cell 89, 575–585. - PubMed
-
- Coursol, S., Fan, L.M., Le Stunff, H., Spiegel, S., Gilroy, S., and Assmann, S.M. (2003). Sphingolipid signalling in Arabidopsis guard cells involves heterotrimeric G proteins. Science 273, 1239–1241. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

