The disulfide isomerase Grp58 is a protective factor against prion neurotoxicity
- PMID: 15772339
- PMCID: PMC6725139
- DOI: 10.1523/JNEUROSCI.4090-04.2005
The disulfide isomerase Grp58 is a protective factor against prion neurotoxicity
Abstract
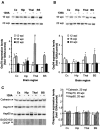
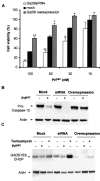
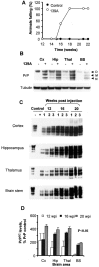
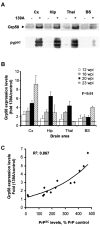
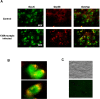
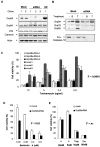
Prion diseases are transmissible neurodegenerative disorders characterized by extensive neuronal apoptosis and accumulation of misfolded prion protein (PrP(SC)). Recent reports indicate that PrP(SC) induces neuronal apoptosis via activation of the endoplasmic reticulum (ER) stress pathway and activation of the ER resident caspase-12. Here, we investigate the relationship between prion replication and induction of ER stress during different stages of the disease in a murine scrapie model. The first alteration observed consists of the upregulation of the ER chaperone of the glucose-regulated protein Grp58, which was detected during the presymptomatic phase and followed closely the formation of PrP(SC). An increase in Grp58 expression correlated with PrP(SC) accumulation at all stages of the disease in different brain areas, suggesting that this chaperone may play an important role in the cellular response to prion infection. Indeed, in vitro studies using N2a neuroblastoma cells demonstrated that inhibition of Grp58 expression with small interfering RNA led to a significant enhancement of PrP(SC) toxicity. Conversely, overexpression of Grp58 protected cells against PrP(SC) toxicity and decreased the rate of caspase-12 activation. Grp58 and PrP were shown to interact by coimmunoprecipitation, observing a higher interaction in cells infected with scrapie prions. Our data indicate that expression of Grp58 is an early cellular response to prion replication, acting as a neuroprotective factor against prion neurotoxicity. Our findings suggest that targeting Grp58 interaction may have applications for developing novel strategies for treatment and early diagnosis of prion diseases.
Figures

References
-
- Barros LF, Stutzin A, Calixto A, Catalan M, Castro J, Hetz C, Hermosilla T (2001) Nonselective cation channels as effectors of free radical-induced rat liver cell necrosis. Hepatology 33: 114-122. - PubMed
-
- Beranger F, Mange A, Goud B, Lehmann S (2002) Stimulation of PrP(C) retrograde transport toward the endoplasmic reticulum increases accumulation of PrP(Sc) in prion-infected cells. J Biol Chem 277: 38972-38977. - PubMed
-
- Breckenridge DG, Germain M, Mathai JP, Nguyen M, Shore GC (2003) Regulation of apoptosis by endoplasmic reticulum pathways. Oncogene 22: 8608-8618. - PubMed
-
- Capellari S, Zaidi SI, Urig CB, Perry G, Smith MA, Petersen RB (1999) Prion protein glycosylation is sensitive to redox change. J Biol Chem 274: 34846-34850. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
