The hypothalamic neuropeptide melanin-concentrating hormone acts in the nucleus accumbens to modulate feeding behavior and forced-swim performance
- PMID: 15772353
- PMCID: PMC6725126
- DOI: 10.1523/JNEUROSCI.1714-04.2005
The hypothalamic neuropeptide melanin-concentrating hormone acts in the nucleus accumbens to modulate feeding behavior and forced-swim performance
Abstract
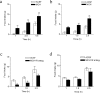
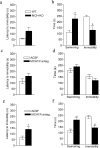
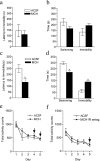
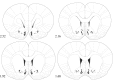
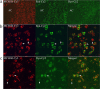
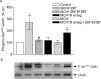
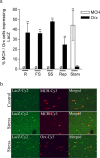
Melanin-concentrating hormone (MCH) is a hypothalamic neuropeptide with a prominent role in feeding and energy homeostasis. The rodent MCH receptor (MCH1R) is highly expressed in the nucleus accumbens shell (AcSh), a region that is important in the regulation of appetitive behavior. Here we establish a role for MCH and MCH1R in mediating a hypothalamic-limbic circuit that regulates feeding and related behaviors. Direct delivery of an MCH1R receptor antagonist to the AcSh blocked feeding and produced an antidepressant-like effect in the forced swim test, whereas intra-AcSh injection of MCH had the opposite effect. Expression studies demonstrated that MCH1R is present in both the enkephalin- and dynorphin-positive medium spiny neurons of the AcSh. Biochemical analysis in AcSh explants showed that MCH signaling blocks dopamine-induced phosphorylation of the AMPA glutamate receptor subunit GluR1 at Ser845. Finally, food deprivation, but not other stressors, stimulated cAMP response element-binding protein-dependent pathways selectively in MCH neurons of the hypothalamus, suggesting that these neurons are responsive to a specific set of physiologically relevant conditions. This work identifies a novel hypothalamic-AcSh circuit that influences appetitive behavior and mediates the antidepressant activity of MCH1R antagonists.
Figures
References
-
- Abbott CR, Kennedy AR, Wren AM, Rossi M, Murphy KG, Seal LJ, Todd JF, Ghatei MA, Small CJ, Bloom SR (2003) Identification of hypothalamic nuclei involved in the orexigenic effect of melanin-concentrating hormone. Endocrinology 144: 3943-3949. - PubMed
-
- Ahima RS, Osei SY (2001) Molecular regulation of eating behavior: new insights and prospects for therapeutic strategies. Trends Mol Med 7: 205-213. - PubMed
-
- Anand BK, Brobeck JR (1951) Localization of a “feeding center” in the hypothalamus of the rat. Proc Soc Exp Biol Med 77: 323-324. - PubMed
-
- Angulo JA, McEwen BS (1994) Molecular aspects of neuropeptide regulation and function in the corpus striatum and nucleus accumbens. Brain Res Brain Res Rev 19: 1-28. - PubMed
-
- Baldo BA, Sadeghian K, Basso AM, Kelley AE (2002) Effects of selective dopamine D1 or D2 receptor blockade within nucleus accumbens subregions on ingestive behavior and associated motor activity. Behav Brain Res 137: 165-177. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
