The opsins
- PMID: 15774036
- PMCID: PMC1088937
- DOI: 10.1186/gb-2005-6-3-213
The opsins
Abstract
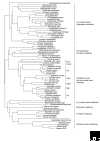
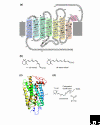
The photosensitive molecule rhodopsin and its relatives consist of a protein moiety - an opsin - and a non-protein moiety - the chromophore retinal. Opsins, which are G-protein-coupled receptors (GPCRs), are found in animals, and more than a thousand have been identified so far. Detailed molecular phylogenetic analyses show that the opsin family is divided into seven subfamilies, which correspond well to functional classifications within the family: the vertebrate visual (transducin-coupled) and non-visual opsin subfamily, the encephalopsin/tmt-opsin subfamily, the Gq-coupled opsin/melanopsin subfamily, the Go-coupled opsin subfamily, the neuropsin subfamily, the peropsin subfamily and the retinal photoisomerase subfamily. The subfamilies diversified before the deuterostomes (including vertebrates) split from the protostomes (most invertebrates), suggesting that a common animal ancestor had multiple opsin genes. Opsins have a seven-transmembrane structure similar to that of other GPCRs, but are distinguished by a lysine residue that is a retinal-binding site in the seventh helix. Accumulated evidence suggests that most opsins act as pigments that activate G proteins in a light-dependent manner in both visual and non-visual systems, whereas a few serve as retinal photoisomerases, generating the chromophore used by other opsins, and some opsins have unknown functions.
Figures
References
-
- Gartner W. Invertebrate visual pigments. In: Stavenga DG, DeGrip WJ, Pugh Jr EN, editor. Handbook of Biological Physics. Vol. 3. Amsterdam: Elsevier Science; 2000. pp. 297–388. A detailed review of invertebrate visual and non-visual opsins.
-
- Takahashi Y, Ebrey TG. Photobiology of retinal proteins. In: Coohill T, Valenzeno D, editor. Photobiology for the 21th Century. Overland Park, Kansas: Valdenmar; 2001. pp. 101–133. A detailed review of vertebrate visual opsins with amino-acid sequence comparisons.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

