Synaptic facilitation and enhanced neuronal excitability in the submucosal plexus during experimental colitis in guinea-pig
- PMID: 15774518
- PMCID: PMC1464458
- DOI: 10.1113/jphysiol.2005.084285
Synaptic facilitation and enhanced neuronal excitability in the submucosal plexus during experimental colitis in guinea-pig
Abstract
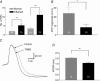
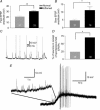
Intestinal secretion is regulated by submucosal neurones of the enteric nervous system. Inflammation of the intestines leads to aberrant secretory activity; therefore we hypothesized that the synaptic and electrical behaviours of submucosal neurones are altered during colitis. To test this hypothesis, we used intracellular microelectrode recording to compare the excitability and synaptic properties of submucosal neurones from normal and trinitrobenzene sulphonic acid (TNBS)-inflamed guinea-pig colons. Inflammation differentially affected the electrophysiological characteristics of the two functional classes of submucosal neurones. AH neurones from inflamed colons were more excitable, had shorter action potential durations and reduced afterhyperpolarizations. Stimulus-evoked fast and slow excitatory postsynaptic potentials (EPSPs) in S neurones were larger during colitis, and the incidence of spontaneous fast EPSPs was increased. In control preparations, fast EPSPs were almost completely blocked by the nicotinic receptor antagonist hexamethonium, whereas fast EPSPs in inflamed S neurones were only partially inhibited by hexamethonium. In inflamed tissues, components of the fast EPSP in S neurones were sensitive to blockade of P2(X) and 5-HT(3) receptors while these antagonists had little effect in control preparations. Control and inflamed S neurones were equally sensitive to brief application of acetylcholine, ATP and 5-HT, suggesting that synaptic facilitation was due to a presynaptic mechanism. Immunoreactivity for 5-HT in the submucosal plexus was unchanged by inflammation; this indicates that altered synaptic transmission was not due to anatomical remodelling of submucosal nerve terminals. This is the first demonstration of alterations in synaptic pharmacology in the enteric nervous system during inflammation.
Figures
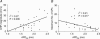
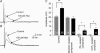

Similar articles
-
Persistent alterations to enteric neural signaling in the guinea pig colon following the resolution of colitis.Am J Physiol Gastrointest Liver Physiol. 2007 Feb;292(2):G482-91. doi: 10.1152/ajpgi.00355.2006. Epub 2006 Sep 28. Am J Physiol Gastrointest Liver Physiol. 2007. PMID: 17008554
-
Enhanced excitability of myenteric AH neurones in the inflamed guinea-pig distal colon.J Physiol. 2003 Mar 1;547(Pt 2):589-601. doi: 10.1113/jphysiol.2002.035147. Epub 2003 Jan 24. J Physiol. 2003. PMID: 12562910 Free PMC article.
-
Cyclooxygenase-2 contributes to dysmotility and enhanced excitability of myenteric AH neurones in the inflamed guinea pig distal colon.J Physiol. 2004 May 15;557(Pt 1):191-205. doi: 10.1113/jphysiol.2004.062174. Epub 2004 Mar 12. J Physiol. 2004. PMID: 15020692 Free PMC article.
-
Colitis-induced neuroplasticity disrupts motility in the inflamed and post-inflamed colon.J Clin Invest. 2015 Mar 2;125(3):949-55. doi: 10.1172/JCI76306. Epub 2015 Mar 2. J Clin Invest. 2015. PMID: 25729851 Free PMC article. Review.
-
Neuronal influence on intestinal transport.J Intern Med Suppl. 1990;732:125-32. doi: 10.1111/j.1365-2796.1990.tb01484.x. J Intern Med Suppl. 1990. PMID: 2166522 Review.
Cited by
-
Depletion of muscularis macrophages ameliorates inflammation-driven dysmotility in murine colitis model.Sci Rep. 2023 Dec 17;13(1):22451. doi: 10.1038/s41598-023-50059-7. Sci Rep. 2023. PMID: 38105266 Free PMC article.
-
Ileitis alters neuronal and enteroendocrine signalling in guinea pig distal colon.Gut. 2007 Feb;56(2):186-94. doi: 10.1136/gut.2006.102780. Epub 2006 Aug 24. Gut. 2007. PMID: 16931576 Free PMC article.
-
Patch clamp recording from enteric neurons in situ.Nat Protoc. 2011 Jan;6(1):15-27. doi: 10.1038/nprot.2010.172. Nat Protoc. 2011. PMID: 21212776
-
Strain-specific genetics, anatomy and function of enteric neural serotonergic pathways in inbred mice.J Physiol. 2009 Feb 1;587(3):567-86. doi: 10.1113/jphysiol.2008.160416. Epub 2008 Dec 8. J Physiol. 2009. PMID: 19064621 Free PMC article.
-
Gut sensory neurons as regulators of neuro-immune-microbial interactions: from molecular mechanisms to precision therapy for IBD/IBS.J Neuroinflammation. 2025 Jul 2;22(1):172. doi: 10.1186/s12974-025-03500-9. J Neuroinflammation. 2025. PMID: 40605050 Free PMC article. Review.
References
-
- Bornstein JC, Furness JB, Kunze WA. Electrophysiological characterization of myenteric neurons: how do classification schemes relate? J Auton Nerv Syst. 1994;48:1–15. - PubMed
-
- De Giorgio R, Guerrini S, Barbara G, Stanghellini V, De Ponti F, Corinaldesi R, Moses PL, Sharkey KA, Mawe GM. Inflammatory neuropathies of the enteric nervous system. Gastroenterology. 2004;126:1872–1883. - PubMed
-
- Ellis LM, Mawe GM. Distribution and chemical coding of cocaine- and amphetamine-regulated transcript peptide (CART) -immunoreactive neurons in the guinea pig bowel. Cell Tissue Res. 2003;312:265–274. 10.1007/s00441-002-0678-9. - DOI - PubMed
-
- Elson CO, Sartor RB, Tennyson GS, Riddell RH. Experimental models of inflammatory bowel disease. Gastroenterology. 1995;109:1344–1367. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

