Defective place cell activity in nociceptin receptor knockout mice with elevated NMDA receptor-dependent long-term potentiation
- PMID: 15774528
- PMCID: PMC1464524
- DOI: 10.1113/jphysiol.2004.081802
Defective place cell activity in nociceptin receptor knockout mice with elevated NMDA receptor-dependent long-term potentiation
Abstract
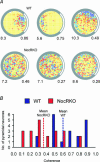
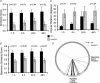
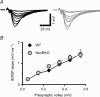
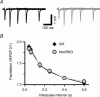
There is growing evidence that NMDA receptor-dependent long-term potentiation (LTP) in the hippocampus mediates the synaptic plasticity that underlies spatial learning and memory. LTP deficiencies correlate well with spatial memory deficits and LTP enhancements may improve spatial memory. In addition, LTP deficiencies are associated with abnormal place cells as expected from the spatial mapping hypothesis of hippocampal function. In contrast, nothing is known on how enhanced NMDA receptor-dependent LTP affects place cells. To address this question we recorded place cells from mice lacking the nociceptin receptor (NOP1/ORL1/OP4) that have enhanced hippocampal LTP. We found that the enhanced LTP was mediated by NMDA receptors, did not require L-type calcium channels, and occurred only when high frequency tetanizing stimulus trains were used. Place cells in nociceptin receptor knockout mice were abnormal in several ways: they were less stable, had noisier positional firing patterns, larger firing fields and higher discharge rates inside and outside the firing fields. Our results suggest that excessive LTP can cause subnormal hippocampal place cell function. The effects of LTP enhancement on place cell function may therefore also depend on molecular details of synaptic plasticity, including the relationship between stimulus frequency and synaptic strength, and not merely on the magnitude of synaptic strength increases. The data have important clinical implications on development of strategies to improve cognitive function.
Figures


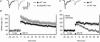
References
-
- Abel T, Nguyen PV, Barad M, Deuel TA, Kandel ER, Bourtchouladze R. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell. 1997;88:615–626. - PubMed
-
- Batschelet E. Circular Statistics in Biology. New York: Academic Press; 1981.
-
- Çavus I, Teyler T. Two forms of long-term potentiation in area CA1 activate different signal transduction cascases. J Neurophysiol. 1996;76:3038–3047. - PubMed
-
- Çavus I, Teyler TJ. NMDA receptor-independent LTP in basal versus apical dendrites of CA1 pyramidal cell in rat hippocampal slice. Hippocampus. 1998;8:373–379. 10.1002/(SICI)1098-1063(1998)8:4<373::AID-HIPO5>3.0.CO;2-I. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

