AMP kinase activation with AICAR simultaneously increases fatty acid and glucose oxidation in resting rat soleus muscle
- PMID: 15774530
- PMCID: PMC1464538
- DOI: 10.1113/jphysiol.2004.081679
AMP kinase activation with AICAR simultaneously increases fatty acid and glucose oxidation in resting rat soleus muscle
Abstract
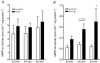
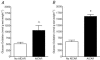
5-Amino-4-imidazolecarboxamide riboside (AICAR), a pharmacological activator of AMP-activated protein kinase (AMPK), acutely stimulates glucose uptake and fatty acid (FA) oxidation in skeletal muscle. However, it is not fully understood whether AICAR-induced changes in glucose oxidation are secondary to changes in FA oxidation (i.e. glucose fatty acid cycle), or what role AMPK may be playing in the regulation of intramuscular triacylglycerol (TAG) esterification and hydrolysis. We examined the acute (60 min) effects of AICAR (2 mm) on FA metabolism, glucose oxidation and pyruvate dehydrogenase (PDH) activation in isolated resting rat soleus muscle strips exposed to two different FA concentrations (low fatty acid, LFA, 0.2 mm; high fatty acid, HFA, 1 mm). AICAR significantly increased AMPK alpha2 activity (+192%; P<0.05) over 60 min, and simultaneously increased both FA (LFA: +33%, P<0.05; HFA: +36%, P<0.05) and glucose (LFA: +105%, P<0.05; HFA: +170, P<0.001) oxidation regardless of FA availability. While there were no changes in TAG esterification, AICAR did increase the ratio of FA partitioned to oxidation relative to TAG esterification (LFA: +15%, P<0.05; HFA: +49%, P<0.05). AICAR had no effect on endogenous TAG hydrolysis and oxidation in resting soleus. The stimulation of glucose oxidation with AICAR was associated with an increase in PDH activation (+126%; P<0.05) but was without effect on pyruvate, an allosteric activator of the PDH complex, suggesting that AMPK may stimulate PDH directly. In conclusion, AMPK appears to be an important regulator of both FA metabolism and glucose oxidation in resting skeletal muscle.
Figures

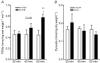
References
-
- Ai H, Ihlemann J, Hellsten Y, Lauritzen HP, Hardie DG, Galbo H, Ploug T. Effect of fiber type and nutritional state on AICAR- and contraction-stimulated glucose transport in rat muscle. Am J Physiol Endocrinol Metab. 2002;282:E1291–E1300. - PubMed
-
- Aschenbach WG, Hirshman MF, Fujii N, Sakamoto K, Howlett KF, Goodyear LJ. Effect of AICAR treatment on glycogen metabolism in skeletal muscle. Diabetes. 2002;51:567–573. - PubMed
-
- Bergeron R, Russell RR, III, Young LH, Ren J-M, Marcucci M, Lee A, Shulman GI. Effect of AMPK activation on muscle glucose metabolism in conscious rats. Am J Physiol Endocrinol Metab. 1999;276:E938–E944. - PubMed
-
- Chen Z-P, McConnell GK, Michell BJ, Snow RJ, Canny BJ, Kemp BE. AMPK signaling in contracting human skeletal muscle: acetyl-CoA carboxylase and NO synthase phosphorylation. Am J Physiol Endocrinol Metab. 2000;279:E1202–E1206. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

