Role of molecular mimicry to HIV-1 peptides in HIV-1-related immunologic thrombocytopenia
- PMID: 15774614
- PMCID: PMC1895171
- DOI: 10.1182/blood-2005-01-0243
Role of molecular mimicry to HIV-1 peptides in HIV-1-related immunologic thrombocytopenia
Abstract
Patients with early HIV-1 infection develop an autoimmune thrombocytopenia in which antibody is directed against an immunodominant epitope of the beta3 (glycoprotein IIIa [GPIIIa]) integrin, GPIIIa49-66. This antibody induces thrombocytopenia by a novel complement-independent mechanism in which platelets are fragmented by antibody-induced generation of H2O2 derived from the interaction of platelet nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and 12-lipoxygenase. To examine whether sharing of epitope between host and parasite may be responsible for this immunodominant epitope, we screened for antibody-reactive peptides capable of inhibiting platelet lysis and oxidation in vitro, using a filamentous phage display 7-mer peptide library. Fourteen of these phage-peptide clones were identified. Five shared close sequence similarity with GPIIIa49-66, as expected. Ten were molecular mimics with close sequence similarity to HIV-1 proteins nef, gag, env, and pol. Seven were synthesized as 10-mers from their known HIV-1 sequence and found to inhibit anti-GPIIIa49-66-induced platelet oxidation/fragmentation in vitro. Three rabbit antibodies raised against these peptides induced platelet oxidation/fragmentation in vitro and thrombocytopenia in vivo when passively transferred into mice. One of the peptides shared a known epitope region with HIV-1 protein nef and was derived from a variant region of the protein. These data provide strong support for molecular mimicry in HIV-1-immunologic thrombocytopenia within polymorphic regions of HIV-1 proteins. A known epitope of nef is particularly incriminated.
Figures
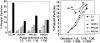
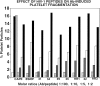
 , irrelevant peptide. The horizontal line, dark gray, white, and black bars refer to molecular Ab/peptide ratios of 1:100, 1:10, 1:5, and 1:2, respectively. Numbers refer to PHC or H clones of Table 1. HR1 refers to H20R1; HR3 refers to H19R3.
, irrelevant peptide. The horizontal line, dark gray, white, and black bars refer to molecular Ab/peptide ratios of 1:100, 1:10, 1:5, and 1:2, respectively. Numbers refer to PHC or H clones of Table 1. HR1 refers to H20R1; HR3 refers to H19R3.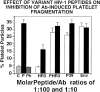


Similar articles
-
Cross-reactive antibodies between HIV-gp120 and platelet gpIIIa (CD61) in HIV-related immune thrombocytopenic purpura.Clin Exp Immunol. 1996 Jan;103(1):19-23. doi: 10.1046/j.1365-2249.1996.917606.x. Clin Exp Immunol. 1996. PMID: 8565280 Free PMC article.
-
Complement-independent, peroxide-induced antibody lysis of platelets in HIV-1-related immune thrombocytopenia.Cell. 2001 Sep 7;106(5):551-61. doi: 10.1016/s0092-8674(01)00477-9. Cell. 2001. PMID: 11551503
-
Specific cross-reaction of anti-dsDNA antibody with platelet integrin GPIIIa49-66.Autoimmunity. 2010 Dec;43(8):682-9. doi: 10.3109/08916934.2010.506207. Epub 2010 Sep 9. Autoimmunity. 2010. PMID: 20828249 Free PMC article.
-
CD4+ T cell epitopes in HIV-1 proteins.Chem Immunol. 1993;56:127-49. Chem Immunol. 1993. PMID: 7680866 Review. No abstract available.
-
HIV-1: seven facets of functional molecular mimicry.Immunol Lett. 1991 May;28(2):91-6; discussion 97-9. doi: 10.1016/0165-2478(91)90104-i. Immunol Lett. 1991. PMID: 1885213
Cited by
-
Anemia and thrombocytopenia in the cohort of HIV-infected adults in northwest Ethiopia: a facility-based cross-sectional study.EJIFCC. 2018 Apr 30;29(1):36-47. eCollection 2018 Apr. EJIFCC. 2018. PMID: 29765285 Free PMC article.
-
Antiretroviral Therapy Improves Acquired Immunodeficiency Syndrome with Systemic Lupus Erythematosus.Life (Basel). 2021 May 21;11(6):463. doi: 10.3390/life11060463. Life (Basel). 2021. PMID: 34063960 Free PMC article.
-
Selected biochemical and hematological abnormalities in Nigerians with human immunodeficiency virus and hepatitis C virus coinfection.Hepat Med. 2011 Jun 30;3:63-8. doi: 10.2147/HMER.S21735. eCollection 2011. Hepat Med. 2011. PMID: 24367222 Free PMC article.
-
Prevalence of HIV-related thrombocytopenia among clients at Mbarara Regional Referral Hospital, Mbarara, southwestern Uganda.J Blood Med. 2015 Apr 10;6:109-13. doi: 10.2147/JBM.S80857. eCollection 2015. J Blood Med. 2015. PMID: 25926763 Free PMC article.
-
Crosstalk Between Platelets and Microbial Pathogens.Front Immunol. 2020 Aug 7;11:1962. doi: 10.3389/fimmu.2020.01962. eCollection 2020. Front Immunol. 2020. PMID: 32849656 Free PMC article. Review.
References
-
- Morris L, Distenfeld A, Amorosi E, Karpatkin S. Autoimmune thrombocytopenic purpura in homosexual men. Ann Intern Med. 1982;96: 714-717. - PubMed
-
- Najean Y, Rain JD. The mechanism of thrombocytopenia in patients with HIV infection. J Lab Clin Med. 1994;123: 415-420. - PubMed
-
- Walsh C, Nardi M, Karpatkin S. On the mechanism of thrombocytopenic purpura in sexually-active homosexual men. N Engl J Med. 1984;311: 635-639. - PubMed
-
- Savona S, Nardi M, Karpatkin S. Thrombocytopenic purpura in narcotics addicts. Ann Intern Med. 1985;102: 737-741. - PubMed
-
- Ratnoff O, Menitove J, Aster R, Lederman M. Coincident classic hemophilia and “idiopathic” thrombocytopenic purpura in patients under treatment with concentrates of anti-hemophilic factor (factor VIII). N Engl J Med. 1983;308: 439-442. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

