N Glycolylation of the nucleotide precursors of peptidoglycan biosynthesis of Mycobacterium spp. is altered by drug treatment
- PMID: 15774877
- PMCID: PMC1065221
- DOI: 10.1128/JB.187.7.2341-2347.2005
N Glycolylation of the nucleotide precursors of peptidoglycan biosynthesis of Mycobacterium spp. is altered by drug treatment
Abstract
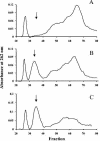
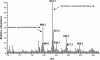
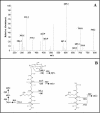
The peptidoglycan of Mycobacterium spp. reportedly has some unique features, including the occurrence of N-glycolylmuramic rather than N-acetylmuramic acid. However, very little is known of the actual biosynthesis of mycobacterial peptidoglycan, including the extent and origin of N glycolylation. In the present work, we have isolated and analyzed muramic acid residues located in peptidoglycan and UDP-linked precursors of peptidoglycan from Mycobacterium tuberculosis and Mycobacterium smegmatis. The muramic acid residues isolated from the mature peptidoglycan of both species were shown to be a mixture of the N-acetyl and N-glycolyl derivatives, not solely the N-glycolylated product as generally reported. The isolated UDP-linked N-acylmuramyl-pentapeptide precursor molecules also contain a mixture of N-acetyl and N-glycolyl muramyl residues in apparent contrast to previous observations in which the precursors isolated after treatment with d-cycloserine consisted entirely of N-glycolyl muropeptides. However, nucleotide-linked peptidoglycan precursors isolated from M. tuberculosis treated with d-cycloserine contained only N-glycolylmuramyl-tripeptide precursors, whereas those from similarly treated M. smegmatis consisted of a mixture of N-glycolylated and N-acetylated residues. The full pentapeptide intermediate, isolated following vancomycin treatment of M. smegmatis, consisted of the N-glycolyl derivative only, whereas the corresponding M. tuberculosis intermediate was a mixture of both the N-glycolyl and N-acetyl products. Thus, treatment with vancomycin and d-cylcoserine not only caused an accumulation of nucleotide-linked intermediate compounds but also altered their glycolylation status, possibly by altering the normal equilibrium maintained by de novo biosynthesis and peptidoglycan recycling.
Figures

Similar articles
-
Identification of the namH gene, encoding the hydroxylase responsible for the N-glycolylation of the mycobacterial peptidoglycan.J Biol Chem. 2005 Jan 7;280(1):326-33. doi: 10.1074/jbc.M411006200. Epub 2004 Nov 2. J Biol Chem. 2005. PMID: 15522883
-
[Study of the formation of N-glycolylmuramic acid from Nocardia asteroides (author's transl)].Biochim Biophys Acta. 1976 Feb 24;421(2):395-405. doi: 10.1016/0304-4165(76)90305-6. Biochim Biophys Acta. 1976. PMID: 1252474 French.
-
Unique structural features of the peptidoglycan of Mycobacterium leprae.J Bacteriol. 2008 Jan;190(2):655-61. doi: 10.1128/JB.00982-07. Epub 2007 Nov 16. J Bacteriol. 2008. PMID: 18024514 Free PMC article.
-
Molecular basis underlying Mycobacterium tuberculosis D-cycloserine resistance. Is there a role for ubiquinone and menaquinone metabolic pathways?Expert Opin Ther Targets. 2014 Jun;18(6):691-701. doi: 10.1517/14728222.2014.902937. Epub 2014 Apr 29. Expert Opin Ther Targets. 2014. PMID: 24773568 Review.
-
Biosynthesis of the arabinogalactan-peptidoglycan complex of Mycobacterium tuberculosis.Glycobiology. 2001 Sep;11(9):107R-118R. doi: 10.1093/glycob/11.9.107r. Glycobiology. 2001. PMID: 11555614 Review.
Cited by
-
Subfamily-specific adaptations in the structures of two penicillin-binding proteins from Mycobacterium tuberculosis.PLoS One. 2014 Dec 31;9(12):e116249. doi: 10.1371/journal.pone.0116249. eCollection 2014. PLoS One. 2014. PMID: 25551456 Free PMC article.
-
Synthesis of Bacterial-Derived Peptidoglycan Cross-Linked Fragments.J Org Chem. 2020 Dec 18;85(24):16243-16253. doi: 10.1021/acs.joc.0c01852. Epub 2020 Oct 27. J Org Chem. 2020. PMID: 33108204 Free PMC article.
-
Bacterial Strategies to Preserve Cell Wall Integrity Against Environmental Threats.Front Microbiol. 2018 Aug 31;9:2064. doi: 10.3389/fmicb.2018.02064. eCollection 2018. Front Microbiol. 2018. PMID: 30233540 Free PMC article. Review.
-
The peptidoglycan of stationary-phase Mycobacterium tuberculosis predominantly contains cross-links generated by L,D-transpeptidation.J Bacteriol. 2008 Jun;190(12):4360-6. doi: 10.1128/JB.00239-08. Epub 2008 Apr 11. J Bacteriol. 2008. PMID: 18408028 Free PMC article.
-
The Gram-Positive Bacterial Cell Wall.Microbiol Spectr. 2019 May;7(3):10.1128/microbiolspec.gpp3-0044-2018. doi: 10.1128/microbiolspec.GPP3-0044-2018. Microbiol Spectr. 2019. PMID: 31124431 Free PMC article. Review.
References
-
- Adam, A., J. F. Petit, J. Wietzerbin-Falszpan, P. Sinay, D. W. Thomas, and E. Lederer. 1969. L'acide N-glycolyl-muramique, contituant des parois de Mycobacterium smegmatis: identification par spectrometrie de masse. FEBS Lett. 4:87-92. - PubMed
-
- Billot-Klein, D., D. Shlaes, D. Bryant, D. Bell, R. Legrand, L. Gutmann, and J. van Heijenoort. 1997. Presence of UDP-N-acetylmuramyl-hexapeptides and -heptapeptides in enterococci and staphylococci after treatment with ramoplanin, tunicamycin, or vancomycin. J. Bacteriol. 179:4684-4688. - PMC - PubMed
-
- Brennan, P. J., and H. Nikaido. 1995. The envelope of mycobacteria. Annu. Rev. Biochem. 64:29-63. - PubMed
-
- Crick, D. C., S. Mahapatra, and P. J. Brennan. 2001. Biosynthesis of the arabinogalactan-peptidoglycan complex of Mycobacterium tuberculosis. Glycobiology 11:107R-118R. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

