N Glycolylation of the nucleotide precursors of peptidoglycan biosynthesis of Mycobacterium spp. is altered by drug treatment
- PMID: 15774877
- PMCID: PMC1065221
- DOI: 10.1128/JB.187.7.2341-2347.2005
N Glycolylation of the nucleotide precursors of peptidoglycan biosynthesis of Mycobacterium spp. is altered by drug treatment
Abstract
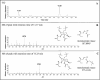
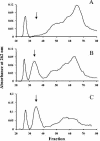
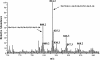
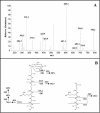
The peptidoglycan of Mycobacterium spp. reportedly has some unique features, including the occurrence of N-glycolylmuramic rather than N-acetylmuramic acid. However, very little is known of the actual biosynthesis of mycobacterial peptidoglycan, including the extent and origin of N glycolylation. In the present work, we have isolated and analyzed muramic acid residues located in peptidoglycan and UDP-linked precursors of peptidoglycan from Mycobacterium tuberculosis and Mycobacterium smegmatis. The muramic acid residues isolated from the mature peptidoglycan of both species were shown to be a mixture of the N-acetyl and N-glycolyl derivatives, not solely the N-glycolylated product as generally reported. The isolated UDP-linked N-acylmuramyl-pentapeptide precursor molecules also contain a mixture of N-acetyl and N-glycolyl muramyl residues in apparent contrast to previous observations in which the precursors isolated after treatment with d-cycloserine consisted entirely of N-glycolyl muropeptides. However, nucleotide-linked peptidoglycan precursors isolated from M. tuberculosis treated with d-cycloserine contained only N-glycolylmuramyl-tripeptide precursors, whereas those from similarly treated M. smegmatis consisted of a mixture of N-glycolylated and N-acetylated residues. The full pentapeptide intermediate, isolated following vancomycin treatment of M. smegmatis, consisted of the N-glycolyl derivative only, whereas the corresponding M. tuberculosis intermediate was a mixture of both the N-glycolyl and N-acetyl products. Thus, treatment with vancomycin and d-cylcoserine not only caused an accumulation of nucleotide-linked intermediate compounds but also altered their glycolylation status, possibly by altering the normal equilibrium maintained by de novo biosynthesis and peptidoglycan recycling.
Figures

References
-
- Adam, A., J. F. Petit, J. Wietzerbin-Falszpan, P. Sinay, D. W. Thomas, and E. Lederer. 1969. L'acide N-glycolyl-muramique, contituant des parois de Mycobacterium smegmatis: identification par spectrometrie de masse. FEBS Lett. 4:87-92. - PubMed
-
- Billot-Klein, D., D. Shlaes, D. Bryant, D. Bell, R. Legrand, L. Gutmann, and J. van Heijenoort. 1997. Presence of UDP-N-acetylmuramyl-hexapeptides and -heptapeptides in enterococci and staphylococci after treatment with ramoplanin, tunicamycin, or vancomycin. J. Bacteriol. 179:4684-4688. - PMC - PubMed
-
- Brennan, P. J., and H. Nikaido. 1995. The envelope of mycobacteria. Annu. Rev. Biochem. 64:29-63. - PubMed
-
- Crick, D. C., S. Mahapatra, and P. J. Brennan. 2001. Biosynthesis of the arabinogalactan-peptidoglycan complex of Mycobacterium tuberculosis. Glycobiology 11:107R-118R. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

