Structural basis of NEDD8 ubiquitin discrimination by the deNEDDylating enzyme NEDP1
- PMID: 15775960
- PMCID: PMC1142549
- DOI: 10.1038/sj.emboj.7600628
Structural basis of NEDD8 ubiquitin discrimination by the deNEDDylating enzyme NEDP1
Abstract
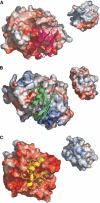
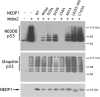
NEDD8 (neural precursor cell expressed developmentally downregulated gene 8)-specific protease NEDP1 processes preNEDD8 to its mature form and deconjugates NEDD8 from substrates such as p53 and cullins. Although NEDD8 and ubiquitin are highly related in sequence and structure, their attachment to a protein leads to different biological effects. It is therefore critical that NEDP1 discriminates between NEDD8 and ubiquitin, and this requires remarkable precision in molecular recognition. To determine the basis of this specificity, we have determined the crystal structure of NEDP1 in isolation and in a transition state complex with NEDD8. This reveals that NEDP1 is a cysteine protease of the Ulp family. Binding of NEDD8 induces a dramatic conformational change in a flexible loop that swings over the C-terminus of NEDD8 locking it into an extended beta-structure optimal for catalysis. Structural, mutational and biochemical studies have identified key residues involved in molecular recognition. A single-residue difference in the C-terminus of NEDD8 and ubiquitin contributes significantly to the ability of NEDP1 to discriminate between them. In vivo analysis indicates that NEDP1 mutants perturb deNEDDylation of the tumour suppressor p53.
Figures

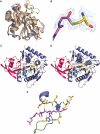


References
-
- Brons-Poulsen J, Petersen NE, Horder M, Kristiansen K (1998) An improved PCR-based method for site directed mutagenesis using megaprimers. Mol Cell Probes 12: 345–348 - PubMed
-
- Chiba T, Tanaka K (2004) Cullin-based ubiquitin ligase and its control by NEDD8-conjugating system. Curr Protein Pept Sci 5: 177–184 - PubMed
-
- Collaborative Computational Project N (1994) The CCP4 suite: programs for protein crystallography. Acta Crystallogr D 50: 760–763 - PubMed
-
- Cope GA, Suh GS, Aravind L, Schwarz SE, Zipursky SL, Koonin EV, Deshaies RJ (2002) Role of predicted metalloprotease motif of Jab1/Csn5 in cleavage of Nedd8 from Cul1. Science 298: 608–611 - PubMed
-
- Deshaies RJ (1999) SCF and Cullin/Ring H2-based ubiquitin ligases. Annu Rev Cell Dev Biol 15: 435–467 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

