Bacterial DNA segregation dynamics mediated by the polymerizing protein ParF
- PMID: 15775965
- PMCID: PMC1142544
- DOI: 10.1038/sj.emboj.7600619
Bacterial DNA segregation dynamics mediated by the polymerizing protein ParF
Abstract
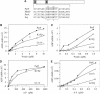
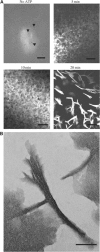
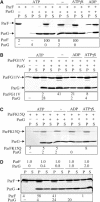
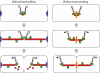
Prokaryotic DNA segregation most commonly involves members of the Walker-type ParA superfamily. Here we show that the ParF partition protein specified by the TP228 plasmid is a ParA ATPase that assembles into extensive filaments in vitro. Polymerization is potentiated by ATP binding and does not require nucleotide hydrolysis. Analysis of mutations in conserved residues of the Walker A motif established a functional coupling between filament dynamics and DNA partitioning. The partner partition protein ParG plays two separable roles in the ParF polymerization process. ParF is unrelated to prokaryotic polymerizing proteins of the actin or tubulin families, but is a homologue of the MinD cell division protein, which also assembles into filaments. The ultrastructures of the ParF and MinD polymers are remarkably similar. This points to an evolutionary parallel between DNA segregation and cytokinesis in prokaryotic cells, and reveals a potential molecular mechanism for plasmid and chromosome segregation mediated by the ubiquitous ParA-type proteins.
Figures





References
-
- Ausmees N, Kuhn JR, Jacobs-Wagner C (2003) The bacterial cytoskeleton: an intermediate filament-like function in cell shape. Cell 115: 705–713 - PubMed
-
- Autret S, Errington J (2003) A role for division-site-selection protein MinD in regulation of internucleoid jumping of Soj (ParA) protein in Bacillus subtilis. Mol Microbiol 47: 159–169 - PubMed
-
- Barillà D, Hayes F (2003) Architecture of the ParF–ParG protein complex involved in procaryotic DNA segregation. Mol Microbiol 49: 487–499 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

