E-cadherin is essential for in vivo epidermal barrier function by regulating tight junctions
- PMID: 15775979
- PMCID: PMC556407
- DOI: 10.1038/sj.emboj.7600605
E-cadherin is essential for in vivo epidermal barrier function by regulating tight junctions
Abstract
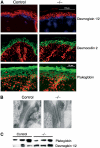
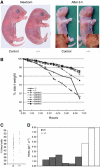
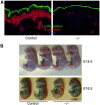
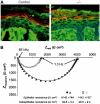
Cadherin adhesion molecules are key determinants of morphogenesis and tissue architecture. Nevertheless, the molecular mechanisms responsible for the morphogenetic contributions of cadherins remain poorly understood in vivo. Besides supporting cell-cell adhesion, cadherins can affect a wide range of cellular functions that include activation of cell signalling pathways, regulation of the cytoskeleton and control of cell polarity. To determine the role of E-cadherin in stratified epithelium of the epidermis, we have conditionally inactivated its gene in mice. Here we show that loss of E-cadherin in the epidermis in vivo results in perinatal death of mice due to the inability to retain a functional epidermal water barrier. Absence of E-cadherin leads to improper localization of key tight junctional proteins, resulting in permeable tight junctions and thus altered epidermal resistance. In addition, both Rac and activated atypical PKC, crucial for tight junction formation, are mislocalized. Surprisingly, our results indicate that E-cadherin is specifically required for tight junction, but not desmosome, formation and this appears to involve signalling rather than cell contact formation.
Figures




References
-
- Anderson JM, Van Itallie CM, Fanning AS (2004) Setting up a selective barrier at the apical junction complex. Curr Opin Cell Biol 16: 140–145 - PubMed
-
- Bilder D, Schober M, Perrimon N (2003) Integrated activity of PDZ protein complexes regulates epithelial polarity. Nat Cell Biol 5: 53–58 - PubMed
-
- Boussadia O, Kutsch S, Hierholzer A, Delmas V, Kemler R (2002) E-cadherin is a survival factor for the lactating mouse mammary gland. Mech Dev 115: 53–62 - PubMed
-
- Braga VM (2002) Cell–cell adhesion and signalling. Curr Opin Cell Biol 14: 546–556 - PubMed
-
- Brandner JM, Kief S, Grund C, Rendl M, Houdek P, Kuhn C, Tschachler E, Franke WW, Moll I (2002) Organization and formation of the tight junction system in human epidermis and cultured keratinocytes. Eur J Cell Biol 81: 253–263 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

