Role of oxidative carbonylation in protein quality control and senescence
- PMID: 15775985
- PMCID: PMC1142534
- DOI: 10.1038/sj.emboj.7600599
Role of oxidative carbonylation in protein quality control and senescence
Abstract
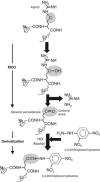
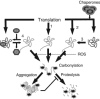
Proteins can become modified by a large number of reactions involving reactive oxygen species. Among these reactions, carbonylation has attracted a great deal of attention due to its irreversible and unrepairable nature. Carbonylated proteins are marked for proteolysis by the proteasome and the Lon protease but can escape degradation and form high-molecular-weight aggregates that accumulate with age. Such carbonylated aggregates can become cytotoxic and have been associated with a large number of age-related disorders, including Parkinson's disease, Alzheimer's disease, and cancer. This review focuses on the generation of and defence against protein carbonyls and speculates on the potential role of carbonylation in protein quality control, cellular deterioration, and senescence.
Figures
References
-
- Agarwal S, Sohal RS (1994) Aging and proteolysis of oxidized proteins. Arch Biochem Biophys 309: 24–28 - PubMed
-
- Aguilaniu H, Gustafsson L, Rigoulet M, Nyström T (2001) Protein oxidation in G0 cells of Saccharomyces cerevisiae depends on the state rather than rate of respiration and is enhanced in pos9 but not yap1 mutants. J Biol Chem 276: 35396–35404 - PubMed
-
- Aguilaniu H, Gustafsson L, Rigoulet M, Nyström T (2003) Asymmetric inheritance of oxidatively damaged proteins during cytokinesis in Saccharomyces cerevisiae: a Sir2p dependent mechanism. Science 299: 1751–1753 - PubMed
-
- Barak Z, Gallant J, Lindsley D, Kwieciszewki B, Heidel D (1996) Enhanced ribosome frameshifting in stationary phase cells. J Mol Biol 263: 140–148 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

