Age-dependent incidence, time course, and consequences of thymic renewal in adults
- PMID: 15776111
- PMCID: PMC1064981
- DOI: 10.1172/JCI22492
Age-dependent incidence, time course, and consequences of thymic renewal in adults
Abstract
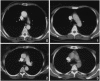
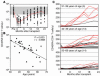
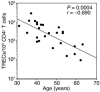
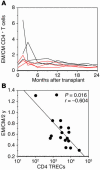
Homeostatic regulation of T cells involves an ongoing balance of new T cell generation, peripheral expansion, and turnover. The recovery of T cells when this balance is disrupted provides insight into the mechanisms that govern homeostasis. In a long-term, single cohort study, we assessed the role of thymic function after autologous transplant in adults, correlating serial computed tomography imaging of thymic size with concurrent measurements of peripheral CD4(+) T cell populations. We established the age-dependent incidence, time course, and duration of thymic enlargement in adults and demonstrated that these changes were correlated with peripheral recovery of naive CD45RA(+)CD62L(+) and signal-joint TCR rearrangement excision circle-bearing CD4(+) populations with broad TCR diversity. Furthermore, we demonstrated that renewed thymopoiesis was critical for the restoration of peripheral CD4(+) T cell populations. This recovery encompassed the recovery of normal CD4(+) T cell numbers, a low ratio of effector to central memory cells, and a broad repertoire of TCR Vbeta diversity among these memory cells. These data define the timeline and consequences of renewal of adult thymopoietic activity at levels able to quantitatively restore peripheral T cell populations. They further suggest that structural thymic regrowth serves as a basis for the regeneration of peripheral T cell populations.
Figures

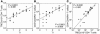



References
-
- Douek DC, Koup RA. Evidence for thymic function in the elderly. Vaccine. 2000;18:1638–1641. - PubMed
-
- Jamieson BD, et al. Generation of functional thymocytes in the human adult. Immunity. 1999;10:569–575. - PubMed
-
- Douek DC, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–695. - PubMed
-
- Schwab R, et al. Expanded CD4+ and CD8+ T cell clones in elderly humans. J. Immunol. 1997;158:4493–4499. - PubMed
-
- Haynes BF, Markert ML, Sempowski GD, Patel DD, Hale LP. The role of the thymus in immune reconstitution in aging, bone marrow transplantation, and HIV-1 infection. Annu. Rev. Immunol. 2000;18:529–560. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

