Ultrastructural analysis of projections to the pulvinar nucleus of the cat. II: Pretectum
- PMID: 15776450
- PMCID: PMC2561319
- DOI: 10.1002/cne.20487
Ultrastructural analysis of projections to the pulvinar nucleus of the cat. II: Pretectum
Abstract
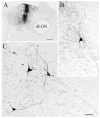
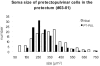
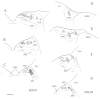
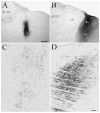
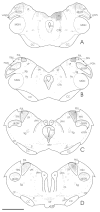
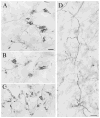
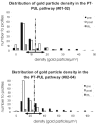
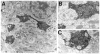
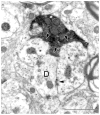
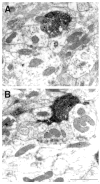
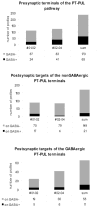
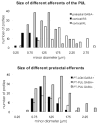
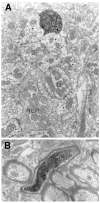
The pretectum (PT) can supply the pulvinar nucleus (PUL), and concomitantly the cortex, with visual motion information through its dense projections to the PUL. We examined the morphology and synaptic targets of pretecto-pulvinar (PT-PUL) terminals labeled by anterograde transport in the cat. By using postembedding immunocytochemical staining for gamma-aminobutyric acid (GABA), we additionally determined whether PT-PUL terminals or their postsynaptic targets were GABAergic. We found that the main projection from the PT to the PUL is an ipsilateral, non-GABAergic projection (72.4%) that primarily contacts thalamocortical cell dendrites (87.6%), and also the dendritic terminals of interneurons (F2 profiles; 12.4%). The PT additionally provides GABAergic innervation to the PUL (27.6% of the ipsilateral projection), which chiefly contacts relay cell dendrites (84.6%) but also GABAergic profiles (15.4%). These GABAergic pretectal terminals are smaller, beaded fibers that likely branch to bilaterally innervate the PUL and dLGN, and possibly other targets. We also examined the neurochemical nature of PT-PUL cells labeled by retrograde transport and found that most are non-GABAergic cells (79%) and devoid of calbindin. Taking existing physiological and our present morphological data into account, we suggest that, in addition to the parietal cortex, the non-GABAergic PT-PUL projection may also strongly influence PUL activity. The GABAergic pretectal fibers, however, may provide a more widespread influence on thalamic activity.
Copyright 2005 Wiley-Liss, Inc.
Figures






References
-
- Baldauf ZB, Wang XP, Wang S, Bickford ME. Pretectotectal pathway: an ultrastructural quantitative analysis in cats. J Comp Neurol. 2003;464:141–158. - PubMed
-
- Ballas I, Hoffmann KP. A correlation between receptive field properties and morphological structures in the pretectum of the cat. J Comp Neurol. 1985;238:417–428. - PubMed
-
- Berman N. Connections of the pretectum in the cat. J Comp Neurol. 1977;174:227–254. - PubMed
-
- Berson DM, Graybiel AM. Parallel thalamic zones in the LP-pulvinar complex of the cat identified by their afferent and efferent connections. Brain Res. 1978;147:139–148. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

