Magnetic resonance imaging of the spine and the sacroiliac joints in ankylosing spondylitis and undifferentiated spondyloarthritis during treatment with etanercept
- PMID: 15778243
- PMCID: PMC1755637
- DOI: 10.1136/ard.2004.032441
Magnetic resonance imaging of the spine and the sacroiliac joints in ankylosing spondylitis and undifferentiated spondyloarthritis during treatment with etanercept
Erratum in
- Ann Rheum Dis. 2005 Nov;64(11):1668
Abstract
Objective: To assess the changes in inflammatory lesions of the spine and the sacroiliac (SI) joints as detected by magnetic resonance imaging (MRI) in patients with ankylosing spondylitis (AS) and undifferentiated spondyloarthritis (uSpA) with predominant axial symptoms during treatment with etanercept.
Methods: MRI of the spine and/or the SI joints of patients with active AS or axial uSpA was performed at baseline (TP0, n = 25), after 6 weeks (TP1, n = 20), and after 24 weeks of continuous treatment with etanercept (TP2, n = 12). T1 weighted spin echo pre -(T1), post-gadolinium (T1/Gd-DTPA) and short tau inversion recovery (STIR) MRI sequences were used to assess chronic and active spinal lesions using the scoring system ASspiMRI. Active and chronic SI lesions were assessed using a simple scoring system.
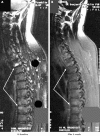
Results: By use of the definite STIR sequence, significant regression of spinal inflammation was already seen already after 6 weeks in the patients treated with etanercept (mean (SD) 11.2 (13.8) at TP0 v 6.8 (7.9) at TP1; p = 0.023) but not in patients treated with placebo. Continuous treatment with etanercept for 24 weeks reduced active spinal changes by 69% (p = 0.012). T1/Gd-DTPA sequences gave similar results. There was only a trend for a decrease of active inflammatory lesions of the SI joints.
Conclusions: Etanercept treatment in patients with active AS and uSpA leads to regression of active inflammatory lesions of the spine as depicted by MRI. The potential role of etanercept on deceleration of chronic spinal changes needs further study.
Figures
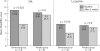



Similar articles
-
Relationship between active inflammatory lesions in the spine and sacroiliac joints and new development of chronic lesions on whole-body MRI in early axial spondyloarthritis: results of the ESTHER trial at week 48.Ann Rheum Dis. 2011 Jul;70(7):1257-63. doi: 10.1136/ard.2010.147033. Epub 2011 May 8. Ann Rheum Dis. 2011. PMID: 21551507 Free PMC article. Clinical Trial.
-
Magnetic resonance imaging examinations of the spine in patients with ankylosing spondylitis before and after therapy with the tumor necrosis factor alpha receptor fusion protein etanercept.Arthritis Rheum. 2005 Apr;52(4):1216-23. doi: 10.1002/art.20977. Arthritis Rheum. 2005. PMID: 15818694 Clinical Trial.
-
Magnetic resonance imaging examinations of the spine in patients with ankylosing spondylitis, before and after successful therapy with infliximab: evaluation of a new scoring system.Arthritis Rheum. 2003 Apr;48(4):1126-36. doi: 10.1002/art.10883. Arthritis Rheum. 2003. PMID: 12687557 Clinical Trial.
-
[Diagnosis and treatment of ankylosing spondylitis (Bechterew disease)].Dtsch Med Wochenschr. 2005 Aug 19;130(33):1882-6. doi: 10.1055/s-2005-871913. Dtsch Med Wochenschr. 2005. PMID: 16118732 Review. German. No abstract available.
-
Change in MRI in patients with spondyloarthritis treated with anti-TNF agents: systematic review of the literature and meta-analysis.Clin Exp Rheumatol. 2021 Mar-Apr;39(2):242-252. doi: 10.55563/clinexprheumatol/fsluso. Epub 2021 Jan 21. Clin Exp Rheumatol. 2021. PMID: 33506749
Cited by
-
Long-term outcome of patients with active ankylosing spondylitis with etanercept-sustained efficacy and safety after seven years.Arthritis Res Ther. 2013;15(3):R67. doi: 10.1186/ar4244. Arthritis Res Ther. 2013. PMID: 23786760 Free PMC article. Clinical Trial.
-
Clinical assessment and outcome research in spondyloarthritis.Curr Rheumatol Rep. 2009 Oct;11(5):334-9. doi: 10.1007/s11926-009-0048-7. Curr Rheumatol Rep. 2009. PMID: 19772828 Review.
-
Successful etanercept therapy for refractory sacroiliitis in a patient with ankylosing spondylitis and mixed connective tissue disease.Yonsei Med J. 2008 Feb 29;49(1):159-62. doi: 10.3349/ymj.2008.49.1.159. Yonsei Med J. 2008. PMID: 18306484 Free PMC article.
-
[Ankylosing spondylitis. Target treatment criteria].Z Rheumatol. 2009 Feb;68(1):30-6. doi: 10.1007/s00393-008-0361-y. Z Rheumatol. 2009. PMID: 19145445 Review. German.
-
Chondrogenesis mediates progression of ankylosing spondylitis through heterotopic ossification.Bone Res. 2021 Mar 17;9(1):19. doi: 10.1038/s41413-021-00140-6. Bone Res. 2021. PMID: 33731675 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

