Resident Th1-like effector memory cells in pulmonary recall responses to Mycobacterium tuberculosis
- PMID: 15778493
- PMCID: PMC2715303
- DOI: 10.1165/rcmb.2005-0060OC
Resident Th1-like effector memory cells in pulmonary recall responses to Mycobacterium tuberculosis
Abstract
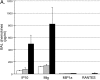
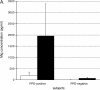
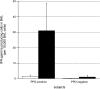
We recently described a model of Th1 recall responses based on segmental antigen challenge with purified protein derivative of Mycobacterium tuberculosis (PPD). Bronchoscopic instillation of 0.5 tuberculin units of PPD resulted in localized lymphocytic inflammation in PPD-positive subjects only. Recruited lymphocytes were predominantly CD4+ and were enriched for cells capable of PPD-specific interferon (IFN)-gamma production. In the current study, we investigated the mechanisms by which this localized recall response is mobilized. Bronchoscopic PPD challenge of skin test-positive subjects resulted in the production of CXCR3 ligands IFN-gamma-inducible protein (IP)-10 and monokine induced by IFN-gamma (Mig), but not of CCR5 ligands macrophage inflammatory protein-1alpha and regulated-upon activation, normal T-cell expressed and secreted, whereas skin test-negative subjects produced none of these chemokines. Baseline bronchoalveolar lavage (BAL) cells of skin test-positive subjects produced IP-10 and Mig in response to in vitro stimulation as well. Because IP-10 and Mig are IFN-gamma-inducible chemokines, these findings suggested that chemokine responses to PPD were facilitated by resident memory cells of the lung. Further studies confirmed that baseline BAL lymphocytes of PPD-positive subjects produce IFN-gamma in response to PPD, and that, compared with peripheral blood, BAL cells are preferentially enriched for PPD-specific lymphocytes. This IFN-gamma production is predominantly a function of CD4+ T cells that display the CD45RO+/CCR7- surface phenotype characteristic of effector memory cells.
Figures





Similar articles
-
Recruitment of antigen-specific Th1-like responses to the human lung following bronchoscopic segmental challenge with purified protein derivative of Mycobacterium tuberculosis.Am J Respir Cell Mol Biol. 2003 Jul;29(1):117-23. doi: 10.1165/rcmb.4840. Am J Respir Cell Mol Biol. 2003. PMID: 12821447
-
Monokine induced by interferon gamma and IFN-gamma response to a fusion protein of Mycobacterium tuberculosis ESAT-6 and CFP-10 in Brazilian tuberculosis patients.Microbes Infect. 2006 Jan;8(1):45-51. doi: 10.1016/j.micinf.2005.05.019. Epub 2005 Jul 22. Microbes Infect. 2006. PMID: 16269263
-
Recruitment of CXCR3+ and CCR5+ T cells and production of interferon-gamma-inducible chemokines in rejecting human arteries.Am J Transplant. 2005 Jun;5(6):1226-36. doi: 10.1111/j.1600-6143.2005.00892.x. Am J Transplant. 2005. PMID: 15888026
-
Hypersensitivity pneumonitis and alpha-chemokines.Clin Ter. 2017 Mar-Apr;168(2):e140-e145. doi: 10.7417/CT.2017.1996. Clin Ter. 2017. PMID: 28383627 Review.
-
The role of chemokines as inflammatory mediators in chronic hepatitis C virus infection.J Viral Hepat. 2007 Oct;14(10):675-87. doi: 10.1111/j.1365-2893.2006.00838.x. J Viral Hepat. 2007. PMID: 17875002 Review.
Cited by
-
Challenges in Developing a Controlled Human Tuberculosis Challenge Model.Curr Top Microbiol Immunol. 2024;445:229-255. doi: 10.1007/82_2022_252. Curr Top Microbiol Immunol. 2024. PMID: 35332386 Review.
-
Host Response to Coccidioides Infection: Fungal Immunity.Front Cell Infect Microbiol. 2020 Nov 11;10:581101. doi: 10.3389/fcimb.2020.581101. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 33262956 Free PMC article. Review.
-
Immune cells in bronchoalveolar lavage fluid of Ugandan adults who resist versus those who develop latent Mycobacterium tuberculosis infection.PLoS One. 2021 Apr 9;16(4):e0249477. doi: 10.1371/journal.pone.0249477. eCollection 2021. PLoS One. 2021. PMID: 33836031 Free PMC article.
-
Mycobacterium tuberculosis-Induced Bronchoalveolar Lavage Gene Expression Signature in Latent Tuberculosis Infection Is Dominated by Pleiotropic Effects of CD4+ T Cell-Dependent IFN-γ Production despite the Presence of Polyfunctional T Cells within the Airways.J Immunol. 2019 Oct 15;203(8):2194-2209. doi: 10.4049/jimmunol.1900230. Epub 2019 Sep 20. J Immunol. 2019. PMID: 31541022 Free PMC article.
-
Tissue resident memory T cells in the respiratory tract.Mucosal Immunol. 2022 Mar;15(3):379-388. doi: 10.1038/s41385-021-00461-z. Epub 2021 Oct 20. Mucosal Immunol. 2022. PMID: 34671115 Free PMC article. Review.
References
-
- Schluger N, Rom WN. The host immune response to tuberculosis. Am J Respir Crit Care Med 1998;157:679–691. - PubMed
-
- Stead W. Management of health care workers after inadvertent exposure to tuberculosis: a guide for the use of preventative therapy. Ann Intern Med 1995;122:906–912. - PubMed
-
- Calhoun W, Jarjour NN, Gleich GJ, Stevens CA, Busse WW. Increased airway inflammation with segmental versus aerosol antigen challenge. Am Rev Respir Dis 1993;147:1465–1471. - PubMed
-
- Jarjour N, Peters SP, Djukanovic R, Calhoun WJ. Investigative use of bronchoscopy in asthma. Am J Respir Crit Care Med 1998;157:692–697. - PubMed
-
- Metzger W, Richerson HB, Worden K, Monick M, Hunninghake GW. Local allergen challenge and bronchoalveolar lavage of allergic asthmatic lungs: description of the model and local airway inflammation. Am Rev Respir Dis 1987;135:433–440. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

