Blockade of HERG cardiac K+ current by antifungal drug miconazole
- PMID: 15778703
- PMCID: PMC1576066
- DOI: 10.1038/sj.bjp.0706095
Blockade of HERG cardiac K+ current by antifungal drug miconazole
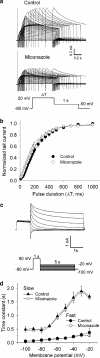
Abstract
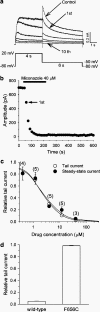
1. Miconazole, an imidazole antifungal agent, is associated with acquired long QT syndrome and ventricular arrhythmias. Miconazole increases the plasma concentration of QT-prolonging drugs by inhibiting the hepatic cytochrome P450 metabolic pathway, but whether it has direct effects on cardiac ion channels has not been elucidated. 2. To determine the mechanism underlying these clinical findings, we investigated the effect of miconazole on human ether-a-go-go-related gene (HERG) K+ channels. 3. HERG channels were heterologously expressed in human embryonic kidney 293 (HEK293) cells and whole-cell currents were recorded using a patch-clamp technique (23 degrees C). 4. Miconazole inhibited HERG peak tail current in a concentration-dependent manner (0.4-40 microM) with an IC50 of 2.1 microM (n=3-5 cells at each concentration, Hill coefficient 1.2). HERG block was not frequency-dependent. It required channel activation, occurred rapidly, and had very slow dissociation properties. 5. The activation curve was shifted in a negative direction (V(1/2): -9.5+/-2.3 mV in controls and -15.3+/-2.4 mV after 4 microM miconazole, P<0.05, n=6). Miconazole did not change other channel kinetics (activation, deactivation, onset of inactivation, recovery from inactivation, steady-state inactivation). 6. The S6 domain mutation, F656C, abolished the inhibitory action of miconazole on HERG current indicating that miconazole preferentially binds to an aromatic amino-acid residue within the pore-S6 region. 7. Our findings indicate that miconazole causes HERG channel block by binding to a common drug receptor, and this involves preferential binding to activated channels. Thus, miconazole prolongs the QT interval by direct inhibition of HERG channels.
Figures



Similar articles
-
Inhibition of cardiac HERG currents by the DNA topoisomerase II inhibitor amsacrine: mode of action.Br J Pharmacol. 2004 Jun;142(3):485-94. doi: 10.1038/sj.bjp.0705795. Epub 2004 May 17. Br J Pharmacol. 2004. PMID: 15148258 Free PMC article.
-
Inhibition of the HERG channel by droperidol depends on channel gating and involves the S6 residue F656.Anesth Analg. 2008 Apr;106(4):1161-70, table of contents. doi: 10.1213/ane.0b013e3181684974. Anesth Analg. 2008. Retraction in: Anesth Analg. 2008 Sep;107(3):1040. doi: 10.1213/ane.0b013e31817e7b40. PMID: 18349188 Retracted.
-
Mesoridazine: an open-channel blocker of human ether-a-go-go-related gene K+ channel.J Mol Cell Cardiol. 2004 Jan;36(1):151-60. doi: 10.1016/j.yjmcc.2003.10.017. J Mol Cell Cardiol. 2004. PMID: 14734057
-
Structural determinants for high-affinity block of hERG potassium channels.Novartis Found Symp. 2005;266:136-50; discussion 150-8. Novartis Found Symp. 2005. PMID: 16050266 Review.
-
Physicochemical basis for binding and voltage-dependent block of hERG channels by structurally diverse drugs.Novartis Found Symp. 2005;266:159-66; discussion 166-70. Novartis Found Symp. 2005. PMID: 16050267 Review.
Cited by
-
Protection of pancreatic islets from oxidative cell death by a peripherally-active morphinan with increased drug safety.Mol Metab. 2023 Sep;75:101775. doi: 10.1016/j.molmet.2023.101775. Epub 2023 Jul 12. Mol Metab. 2023. PMID: 37451343 Free PMC article.
-
Evaluation of combination therapy for Burkholderia cenocepacia lung infection in different in vitro and in vivo models.PLoS One. 2017 Mar 1;12(3):e0172723. doi: 10.1371/journal.pone.0172723. eCollection 2017. PLoS One. 2017. PMID: 28248999 Free PMC article.
-
The Anti-Fungal Activity of Nitropropenyl Benzodioxole (NPBD), a Redox-Thiol Oxidant and Tyrosine Phosphatase Inhibitor.Antibiotics (Basel). 2022 Sep 2;11(9):1188. doi: 10.3390/antibiotics11091188. Antibiotics (Basel). 2022. PMID: 36139967 Free PMC article.
-
Torsades de Pointes ventricular tachycardia in a pediatric patient treated with fluconazole.Pediatr Cardiol. 2008 Jan;29(1):210-3. doi: 10.1007/s00246-007-9076-0. Epub 2007 Sep 12. Pediatr Cardiol. 2008. PMID: 17849073
-
Escitalopram block of hERG potassium channels.Naunyn Schmiedebergs Arch Pharmacol. 2014 Jan;387(1):23-32. doi: 10.1007/s00210-013-0911-y. Epub 2013 Sep 18. Naunyn Schmiedebergs Arch Pharmacol. 2014. PMID: 24045971
References
-
- ABBOTT G.W., SESTI F., SPLAWSKI I., BUCK M.E., LEHMANN M.H., TIMOTHY K.W., KEATING M.T., GOLDSTEIN S.A.N. MiRP1 forms IKr potassium channels with HERG and is associated with cardiac arrhythmia. Cell. 1999;97:175–187. - PubMed
-
- ALBENGRES E., LE LOUET H., TILLEMENT J.P. Systemic antifungal agents. Drug interactions of clinical significance. Drug Saf. 1998;18:83–97. - PubMed
-
- CARMELIET E. Voltage- and time-dependent block of the delayed K+ current in cardiac myocytes by dofetilide. J. Pharmacol. Exp. Ther. 1992;262:809–817. - PubMed
-
- CARMELIET E. Mechanisms and control of repolarization. Eur. Heart J. 1993;14 Suppl H:3–13. - PubMed
-
- COLEY K.C., CRAIN J.L. Miconazole-induced fatal dysrhythmia. Pharmacotherapy. 1997;17:379–382. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

