Synergistic and additive interactions of the cannabinoid agonist CP55,940 with mu opioid receptor and alpha2-adrenoceptor agonists in acute pain models in mice
- PMID: 15778704
- PMCID: PMC1576059
- DOI: 10.1038/sj.bjp.0706045
Synergistic and additive interactions of the cannabinoid agonist CP55,940 with mu opioid receptor and alpha2-adrenoceptor agonists in acute pain models in mice
Abstract
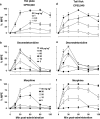
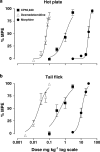
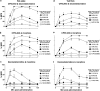
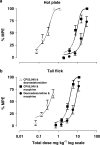
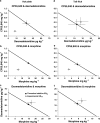
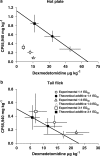
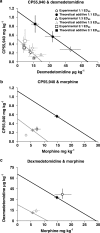
1. Cannabinoid receptor agonists elicit analgesic effects in acute and chronic pain states via spinal and supraspinal pathways. We investigated whether the combination of a cannabinoid agonist with other classes of antinociceptive drugs exerted supra-additive (synergistic) or additive effects in acute pain models in mice. 2. The interactions between the cannabinoid agonist CP55,940, alpha2-adrenoceptor agonist dexmedetomidine and mu-opioid receptor agonist morphine were evaluated by isobolographic analysis of antinociception in hot plate (55 degrees C) and tail flick assays in conscious male Swiss mice. Drug interactions were examined by administering fixed-ratio combinations of agonists (s.c.) in 1:1, 3:1 and 1:3 ratios of their respective ED50 fractions. 3. CP55,940, dexmedetomidine and morphine all caused dose-dependent antinociception. In the hot plate and tail flick assays, ED50 values (mg kg(-1)) were CP55,940 1.13 and 0.51, dexmedetomidine 0.066 and 0.023, and morphine 29.4 and 11.3, respectively. Synergistic interactions existed between CP55,940 and dexmedetomidine in the hot plate assay, and CP55,940 and morphine in both assays. Additive interactions were found for CP55,940 and dexmedetomidine in the tail flick assay, and dexmedetomidine and morphine in both assays. 4. Thus, an alpha2-adrenoceptor agonist or mu opioid receptor agonist when combined with a cannabinoid receptor agonist showed significant synergy in antinociception in the hot plate test. However, for the tail flick nociceptive response to heat, only cannabinoid and mu opioid receptor antinociceptive synergy was demonstrated. If these results translate to humans, then prudent selection of dose and receptor-specific agonists may allow an improved therapeutic separation from unwanted side effects.
Figures
References
-
- ASHTON C.H. Pharmacology and effects of cannabis: a brief review. Br. J. Psychiatry. 2001;178:101–106. - PubMed
-
- BEHBEHANI M.M. Functional characteristics of the midbrain periaqueductal gray. Prog. Neurobiol. 1995;46:575–605. - PubMed
-
- CHAPERON F., THIEBOT M.H. Behavioral effects of cannabinoid agents in animals. Crit. Rev. Neurobiol. 1999;13:243–281. - PubMed
-
- CICHEWICZ D.L., MCCARTHY E.A. Antinociceptive synergy between Δ9-tetrahydrocannabinol and opioids after oral administration. J. Pharmacol. Exp. Ther. 2003;304:1010–1015. - PubMed
-
- COURSIN D.B., MACCIOLI G.A. Dexmedetomidine. Curr. Opin. Crit. Care. 2001;7:221–226. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

