CB1 cannabinoid receptor-mediated modulation of food intake in mice
- PMID: 15778743
- PMCID: PMC1576140
- DOI: 10.1038/sj.bjp.0706157
CB1 cannabinoid receptor-mediated modulation of food intake in mice
Abstract
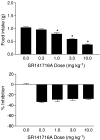
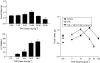
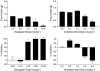
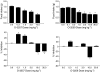
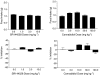
1 Marijuana's appetite-increasing effects have long been known. Recent research suggests that the CB(1) cannabinoid receptor antagonist SR141716A may suppress appetite. This study represents a further, systematic investigation of the role of CB(1) cannabinoid receptors in the pharmacological effects of cannabinoids on food intake. 2 Mice were food-restricted for 24 h and then allowed access to their regular rodent chow for 1 h. Whereas the CB(1) antagonist SR141716A dose-dependently decreased food consumption at doses that did not affect motor activity, Delta(9)-tetrahydrocannabinol (Delta(9)-THC) increased food consumption at doses that had no effect on motor activity. O-3259 and O-3257, structural analogs of SR141716A, produced effects similar to those of the parent compound. 3 Amphetamine (a known anorectic) and diazepam (a benzodiazepine and CNS depressant) decreased food consumption, but only at doses that also increased or decreased motor activity, respectively. The CB(2) cannabinoid receptor antagonist SR144528 and the nonpsychoactive cannabinoid cannabidiol did not affect food intake nor activity. 4 SR141716A decreased feeding in wild-type mice, but lacked pharmacological activity in CB(1) knockout mice; however, basal food intake was lower in CB(1) knockout mice. Amphetamine decreased feeding in both mouse genotypes. 5 These results suggest that SR141716A may affect the actions of endogenous cannabinoids in regulating appetite or that it may have effects of its own aside from antagonism of cannabinoid effects (e.g., decreased feeding behavior and locomotor stimulation). In either case, these results strongly suggest that CB(1) receptors may play a role in regulation of feeding behavior.
Figures

Similar articles
-
Structural analogs of pyrazole and sulfonamide cannabinoids: effects on acute food intake in mice.Eur J Pharmacol. 2012 Nov 15;695(1-3):62-70. doi: 10.1016/j.ejphar.2012.08.019. Epub 2012 Sep 6. Eur J Pharmacol. 2012. PMID: 22975289 Free PMC article.
-
Evidence for an interaction between CB1 cannabinoid and oxytocin receptors in food and water intake.Neuropharmacology. 2004 Sep;47(4):593-603. doi: 10.1016/j.neuropharm.2004.06.002. Neuropharmacology. 2004. PMID: 15380376
-
Evidence for an interaction between CB1 cannabinoid and melanocortin MCR-4 receptors in regulating food intake.Endocrinology. 2004 Jul;145(7):3224-31. doi: 10.1210/en.2004-0059. Epub 2004 Mar 19. Endocrinology. 2004. PMID: 15033920
-
Central versus peripheral antagonism of cannabinoid CB1 receptor in obesity: effects of LH-21, a peripherally acting neutral cannabinoid receptor antagonist, in Zucker rats.J Neuroendocrinol. 2008 May;20 Suppl 1:116-23. doi: 10.1111/j.1365-2826.2008.01693.x. J Neuroendocrinol. 2008. PMID: 18426510 Review.
-
Cannabinoid CB1 receptor inverse agonists and neutral antagonists: effects on food intake, food-reinforced behavior and food aversions.Physiol Behav. 2007 Jul 24;91(4):383-8. doi: 10.1016/j.physbeh.2007.04.013. Epub 2007 Apr 14. Physiol Behav. 2007. PMID: 17521686 Free PMC article. Review.
Cited by
-
Elucidating cannabinoid biology in zebrafish (Danio rerio).Gene. 2015 Oct 10;570(2):168-79. doi: 10.1016/j.gene.2015.07.036. Epub 2015 Jul 17. Gene. 2015. PMID: 26192460 Free PMC article. Review.
-
Characterization of the effects of cannabinoid receptor deletion on energy metabolism in female C57BL mice.Front Endocrinol (Lausanne). 2024 Jun 19;15:1386230. doi: 10.3389/fendo.2024.1386230. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38962676 Free PMC article.
-
Mice Expressing a "Hyper-Sensitive" Form of the Cannabinoid Receptor 1 (CB1) Are Neither Obese Nor Diabetic.PLoS One. 2016 Aug 8;11(8):e0160462. doi: 10.1371/journal.pone.0160462. eCollection 2016. PLoS One. 2016. PMID: 27501235 Free PMC article.
-
Physical activity and the endocannabinoid system: an overview.Cell Mol Life Sci. 2014 Jul;71(14):2681-98. doi: 10.1007/s00018-014-1575-6. Epub 2014 Feb 14. Cell Mol Life Sci. 2014. PMID: 24526057 Free PMC article. Review.
-
Potential anxiogenic effects of cannabinoid CB1 receptor antagonists/inverse agonists in rats: comparisons between AM4113, AM251, and the benzodiazepine inverse agonist FG-7142.Eur Neuropsychopharmacol. 2010 Feb;20(2):112-22. doi: 10.1016/j.euroneuro.2009.11.002. Epub 2009 Dec 16. Eur Neuropsychopharmacol. 2010. PMID: 20015619 Free PMC article.
References
-
- ARNONE M., MARUANI J., CHAPERON F., THIEBOT M.H., PONCELET M., SOUBRIE P., LE FUR G. Selective inhibition of sucrose and ethanol intake by SR 141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology. 1997;132:104–106. - PubMed
-
- AVRAHAM Y., BEN-SHUSHAN D., BREUER A., ZOLOTAREV O., OKON A., FINK N., KATZ V., BERRY E.M. Very low doses of Δ8-THC increase food consumption and alter neurotransmitter levels following weight loss. Pharmacol. Biochem. Behav. 2004;77:675–684. - PubMed
-
- BEAL J.E., OLSON R., LAUBENSTEIN L., MORALES J.O., BELLMAN P., YANGCO B., LEFKOWITZ L., PLASSE T.F., SHEPARD K.V. Dronabinol as a treatment for anorexia associated with weight loss in patients with AIDS. J. Pain Symptom Manage. 1995;10:89–97. - PubMed
-
- CHIESI M., HUPPERTZ C., HOFBAUER K.G. Pharmacotherapy of obesity: targets and perspectives. Trends Pharmacol. Sci. 2001;22:247–254. - PubMed
-
- COTA D., MARSICANO G., LUTZ B., VICENNATI V., STALLA G.K., PASQUALI R., PAGOTTO U. Endogenous cannabinoid system as a modulator of food intake. Int. J. Obes. 2003a;27:289–301. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

