Mechanical strain injury increases intracellular sodium and reverses Na+/Ca2+ exchange in cortical astrocytes
- PMID: 15779085
- PMCID: PMC2996279
- DOI: 10.1002/glia.20183
Mechanical strain injury increases intracellular sodium and reverses Na+/Ca2+ exchange in cortical astrocytes
Abstract
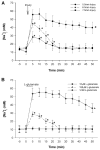
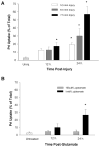
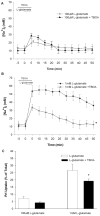
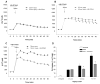
Traditionally, astrocytes have been considered less susceptible to injury than neurons. Yet, we have recently shown that astrocyte death precedes neuronal death in a rat model of traumatic brain injury (TBI) (Zhao et al.: Glia 44:140-152, 2003). A main mechanism hypothesized to contribute to cellular injury and death after TBI is elevated intracellular calcium ([Ca2+]i). Since calcium regulation is also influenced by regulation of intracellular sodium ([Na+]i), we used an in vitro model of strain-induced traumatic injury and live-cell fluorescent digital imaging to investigate alterations in [Na+]i in cortical astrocytes after injury. Changes in [Na+]i, or [Ca2+]i were monitored after mechanical injury or L-glutamate exposure by ratiometric imaging of sodium-binding benzofuran isophthalate (SBFI-AM), or Fura-2-AM, respectively. Mechanical strain injury or exogenous glutamate application produced increases in [Na+]i that were dependent on the severity of injury or concentration. Injury-induced increases in [Na+]i were significantly reduced, but not completely eliminated, by inhibition of glutamate uptake by DL-threo-beta-benzyloxyaspartate (TBOA). Blockade of sodium-dependent calcium influx through the sodium-calcium exchanger with 2-[2-[4-(4-Nitrobenzyloxy)phenyl]ethyl]isothiourea mesylate (KB-R7943) reduced [Ca2+]i after injury. KB-R7943 also reduced astrocyte death after injury. These findings suggest that in astrocytes subjected to mechanical injury or glutamate excitotoxicity, increases in intracellular Na+ may be a critical component in the injury cascade and a therapeutic target for reduction of lasting deficits after traumatic brain injury.
Copyright (c) 2005 Wiley-Liss, Inc.
Figures


References
-
- Ahmed SM, Rzigalinski BA, Willoughby KA, Sitterding HA, Ellis EF. Stretch-induced injury alters mitochondrial membrane potential and cellular ATP in cultured astrocytes and neurons. J Neurochem. 2000;74:1951–1960. - PubMed
-
- Amruthesh SC, Boerschel MF, McKinney JS, Willoughby KA, Ellis EF. Metabolism of arachidonic acid to epoxyeicosatrienoic acids, hydroxyeicosatetraenoic acids, and prostaglandins in cultured rat hippocampal astrocytes. J Neurochem. 1993;61:150–159. - PubMed
-
- Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000;32:1–14. - PubMed
-
- Arakawa N, Sakaue M, Yokoyama I, Hashimoto H, Koyama Y, Baba A, Matsuda T. KB-R7943 inhibits store-operated Ca2+ entry in cultured neurons and astrocytes. Biochem Biophys Res Commun. 2000;279:354–357. - PubMed
-
- Araque A, Sanzgiri RP, Parpura V, Haydon PG. Astrocyte-induced modulation of synaptic transmission. Can J Physiol Pharmacol. 1999;77:699–706. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

