Platelet activation leads to activation and propagation of the complement system
- PMID: 15781579
- PMCID: PMC2213112
- DOI: 10.1084/jem.20041497
Platelet activation leads to activation and propagation of the complement system
Abstract
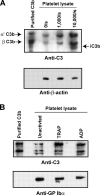
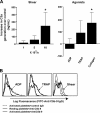
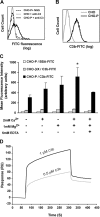
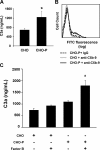
Inflammation and thrombosis are two responses that are linked through a number of mechanisms, one of them being the complement system. Various proteins of the complement system interact specifically with platelets, which, in turn, activates them and promotes thrombosis. In this paper, we show that the converse is also true: activated platelets can activate the complement system. As assessed by flow cytometry and immunoblotting, C3 deposition increased on the platelet surface upon cell activation with different agonists. Activation of the complement system proceeded to its final stages, which was marked by the increased generation of the anaphylotoxin C3a and the C5b-9 complex. We identified P-selectin as a C3b-binding protein, and confirmed by surface plasmon resonance binding that these two proteins interact specifically with a dissociation constant of 1 microM. Using heterologous cells expressing P-selectin, we found that P-selectin alone is sufficient to activate the complement system, marked by increases in C3b deposition, C3a generation, and C5b-9 formation. In summary, we have found that platelets are capable of activating the complement system, and have identified P-selectin as a receptor for C3b capable of initiating complement activation. These findings point out an additional mechanism by which inflammation may localize to sites of vascular injury and thrombosis.
Figures



References
-
- Mastellos, D., and J.D. Lambris. 2002. Complement: more than a “guard” against invading pathogens? Trends Immunol. 23:485–491. - PubMed
-
- Gralnick, H.R., M. Vail, L.P. McKeown, P. Merryman, O. Wilson, I. Chu, and J. Kimball. 1995. Activated platelets in paroxysmal nocturnal haemoglobinuria. Br. J. Haematol. 91:697–702. - PubMed
-
- Qin, X., N. Krumrei, L. Grubissich, M. Dobarro, H. Aktas, G. Perez, and J.A. Halperin. 2003. Deficiency of the mouse complement regulatory protein mCd59b results in spontaneous hemolytic anemia with platelet activation and progressive male infertility. Immunity. 18:217–227. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

