In vivo depletion of lung CD11c+ dendritic cells during allergen challenge abrogates the characteristic features of asthma
- PMID: 15781587
- PMCID: PMC2213109
- DOI: 10.1084/jem.20042311
In vivo depletion of lung CD11c+ dendritic cells during allergen challenge abrogates the characteristic features of asthma
Abstract
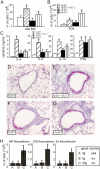
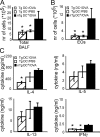
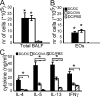
Although dendritic cells (DCs) play an important role in sensitization to inhaled allergens, their function in ongoing T helper (Th)2 cell-mediated eosinophilic airway inflammation underlying bronchial asthma is currently unknown. Here, we show in an ovalbumin (OVA)-driven murine asthma model that airway DCs acquire a mature phenotype and interact with CD4(+) T cells within sites of peribronchial and perivascular inflammation. To study whether DCs contributed to inflammation, we depleted DCs from the airways of CD11c-diphtheria toxin (DT) receptor transgenic mice during the OVA aerosol challenge. Airway administration of DT depleted CD11c(+) DCs and alveolar macrophages and abolished the characteristic features of asthma, including eosinophilic inflammation, goblet cell hyperplasia, and bronchial hyperreactivity. In the absence of CD11c(+) cells, endogenous or adoptively transferred CD4(+) Th2 cells did not produce interleukin (IL)-4, IL-5, and IL-13 in response to OVA aerosol. In CD11c-depleted mice, eosinophilic inflammation and Th2 cytokine secretion were restored by adoptive transfer of CD11c(+) DCs, but not alveolar macrophages. These findings identify lung DCs as key proinflammatory cells that are necessary and sufficient for Th2 cell stimulation during ongoing airway inflammation.
Figures






References
-
- Wills-Karp, M. 1999. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu. Rev. Immunol. 17:255–281. - PubMed
-
- Herrick, C.A., and K. Bottomly. 2003. To respond or not to respond: T cells in allergic asthma. Nat. Rev. Immunol. 3:405–412. - PubMed
-
- Banchereau, J., and R.M. Steinman. 1998. Dendritic cells and the control of immunity. Nature. 392:245–252. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

