Hookworm: "the great infection of mankind"
- PMID: 15783256
- PMCID: PMC1069663
- DOI: 10.1371/journal.pmed.0020067
Hookworm: "the great infection of mankind"
Abstract
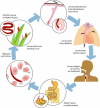
Over the last five years, there has been increasing recognition of the global health importance of hookworm. New international efforts to control the morbidity of hookworm are in progress
Conflict of interest statement
Figures



References
-
- Stoll NR. On endemic hookworm, where do we stand today? Exp Parasitol. 1962;12:241–252. - PubMed
-
- Hotez PJ. The National Institutes of Health roadmap and the developing world. J Investig Med. 2004;52:246–247. - PubMed
-
- Hotez PJ, Brooker S, Bethony J, Bottazzi ME, Loukas A, et al. Current concepts: Hookworm infection. N Engl J Med. 2004;351:799–807. - PubMed
-
- de Silva NR, Brooker S, Hotez PJ, Montresor A, Engels D, et al. Soil-transmitted helminth infections: Updating the global picture. Trends Parasitol. 2003;19:547–551. - PubMed
-
- Hotez PJ. China's hookworms. China Q. 2002;172:1029–1041.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

