Role of YadA, Ail, and Lipopolysaccharide in Serum Resistance of Yersinia enterocolitica Serotype O:3
- PMID: 15784567
- PMCID: PMC1087390
- DOI: 10.1128/IAI.73.4.2232-2244.2005
Role of YadA, Ail, and Lipopolysaccharide in Serum Resistance of Yersinia enterocolitica Serotype O:3
Abstract
Complement attack is a host strategy leading to elimination of pathogens. Yersinia enterocolitica expresses several potential complement resistance factors: the outer membrane proteins YadA and Ail as well as lipopolysaccharide (LPS). To study the contribution of these factors to the survival of Y. enterocolitica serotype O:3 in nonimmune human serum, we constructed 23 mutant strains of Y. enterocolitica O:3 expressing different combinations of YadA, Ail, LPS O antigen, and LPS outer core. Survival of bacteria was analyzed in normal serum (with functional classical, lectin, and alternative complement activation pathways) and EGTA-Mg-treated serum (only alternative pathway functional). Kinetic killing tests revealed that the most potent single-serum resistance factor needed for long-term survival was YadA; Ail was also indispensable, but it provided short-term survival and delayed the bacterial killing. On the contrary, the LPS O antigen and outer core, when in combination with YadA, Ail, or both, had a minor and often negative effect on serum resistance. Bacteria in the exponential phase of growth were more resistant to serum killing than stationary-phase bacteria. After exposing bacteria to EGTA-Mg-treated serum, O antigen could prevent deposition of covalently bound C3b on bacteria at 3 min of incubation, even as a single factor. At later time points (15 and 30 min) it had to be accompanied by YadA, Ail, and outer core. In normal serum, the bacteria were less resistant to C3b deposition. However, no direct correlation between the C3 deposition pattern and bacterial resistance was observed.
Figures


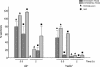

References
-
- Al-Hendy, A., P. Toivanen, and M. Skurnik. 1991. Expression cloning of Yersinia enterocolitica O:3 rfb gene cluster in Escherichia coli K12. Microb. Pathog. 10:47-59. - PubMed
-
- Alitalo, A., T. Meri, T. Chen, H. Lankinen, Z. Z. Cheng, T. S. Jokiranta, I. J. Seppala, P. Lahdenne, P. S. Hefty, D. R. Akins, and S. Meri. 2004. Lysine-dependent multipoint binding of the Borrelia burgdorferi virulence factor outer surface protein E to the C terminus of factor H. J. Immunol. 172:6195-6201. - PubMed
-
- Ausubel, F. M., R. Brent, R. E. Kingston, O. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1987. Current protocols in molecular biology. John Wiley & Sons, New York, N.Y.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

