Involvement of the Escherichia coli O157:H7(pO157) ecf operon and lipid A myristoyl transferase activity in bacterial survival in the bovine gastrointestinal tract and bacterial persistence in farm water troughs
- PMID: 15784583
- PMCID: PMC1087426
- DOI: 10.1128/IAI.73.4.2367-2378.2005
Involvement of the Escherichia coli O157:H7(pO157) ecf operon and lipid A myristoyl transferase activity in bacterial survival in the bovine gastrointestinal tract and bacterial persistence in farm water troughs
Abstract
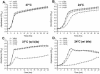
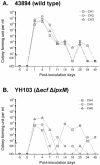
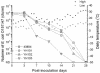
Escherichia coli O157:H7 is an important food-borne pathogen that causes hemorrhagic colitis and the hemolytic-uremic syndrome in humans. Recently, we reported that the pO157 ecf (E. coli attaching and effacing gene-positive conserved fragments) operon is thermoregulated by an intrinsically curved DNA and contains the genes for bacterial surface-associated proteins, including a second copy of lipid A myristoyl transferase, whose chromosomal copy is the lpxM gene product. E. coli O157:H7 survives and persists well in diverse environments from the human and bovine gastrointestinal tracts (GIT) to nutrient-dilute farm water troughs. Transcriptional regulation of the ecf operon by intrinsic DNA curvature and the genetic redundancy of lpxM that is associated with lipid A modification led us to hypothesize that the pO157 ecf operon and lpxM are associated with bacterial survival and persistence in various in vivo and ex vivo environments by optimizing bacterial membrane structure and/or integrity. To test this hypothesis, three isogenic ecf operon and/or lpxM deletion mutants of E. coli O157:H7 ATCC 43894 were constructed and analyzed in vitro and in vivo. The results showed that a double mutant carrying deletions in the ecf and lpxM genes had an altered lipid A structure and membrane fatty acid composition, did not survive passage through the bovine GIT, did not persist well in farm water troughs, had increased susceptibility to a broad spectrum of antibiotics and detergents, and had impaired motility. Electron microscopic analyses showed gross changes in bacterial membrane structure.
Figures





Similar articles
-
Thermoregulation of the Escherichia coli O157:H7 pO157 ecf operon and lipid A myristoyl transferase activity involves intrinsically curved DNA.Mol Microbiol. 2004 Jan;51(2):419-35. doi: 10.1046/j.1365-2958.2003.03827.x. Mol Microbiol. 2004. PMID: 14756783
-
Characterization of an Escherichia coli O157:H7 plasmid O157 deletion mutant and its survival and persistence in cattle.Appl Environ Microbiol. 2007 Apr;73(7):2037-47. doi: 10.1128/AEM.02643-06. Epub 2007 Feb 2. Appl Environ Microbiol. 2007. PMID: 17277224 Free PMC article.
-
Phenotypic diversity of Escherichia coli O157:H7 strains associated with the plasmid O157.J Microbiol. 2010 Jun;48(3):347-57. doi: 10.1007/s12275-010-9228-4. Epub 2010 Jun 23. J Microbiol. 2010. PMID: 20571953 Free PMC article.
-
The ecological habitat and transmission of Escherichia coli O157:H7.FEMS Microbiol Lett. 2013 Apr;341(1):1-12. doi: 10.1111/1574-6968.12078. Epub 2013 Jan 29. FEMS Microbiol Lett. 2013. PMID: 23305397 Review.
-
Perspectives on super-shedding of Escherichia coli O157:H7 by cattle.Foodborne Pathog Dis. 2015 Feb;12(2):89-103. doi: 10.1089/fpd.2014.1829. Epub 2014 Dec 16. Foodborne Pathog Dis. 2015. PMID: 25514549 Review.
Cited by
-
Disruption of rcsB by a duplicated sequence in a curli-producing Escherichia coli O157:H7 results in differential gene expression in relation to biofilm formation, stress responses and metabolism.BMC Microbiol. 2017 Mar 8;17(1):56. doi: 10.1186/s12866-017-0966-x. BMC Microbiol. 2017. PMID: 28274217 Free PMC article.
-
Metallohelices that kill Gram-negative pathogens using intracellular antimicrobial peptide pathways.Chem Sci. 2019 Sep 5;10(42):9708-9720. doi: 10.1039/c9sc03532j. eCollection 2019 Nov 14. Chem Sci. 2019. PMID: 32015803 Free PMC article.
-
Extensive genomic diversity and selective conservation of virulence-determinants in enterohemorrhagic Escherichia coli strains of O157 and non-O157 serotypes.Genome Biol. 2007;8(7):R138. doi: 10.1186/gb-2007-8-7-r138. Genome Biol. 2007. PMID: 17711596 Free PMC article.
-
Inactivation of enterohemorrhagic Escherichia coli in rumen content- or feces-contaminated drinking water for cattle.Appl Environ Microbiol. 2006 May;72(5):3268-73. doi: 10.1128/AEM.72.5.3268-3273.2006. Appl Environ Microbiol. 2006. PMID: 16672466 Free PMC article.
-
Improvement of sabinene tolerance of Escherichia coli using adaptive laboratory evolution and omics technologies.Biotechnol Biofuels. 2020 Apr 24;13:79. doi: 10.1186/s13068-020-01715-x. eCollection 2020. Biotechnol Biofuels. 2020. PMID: 32346395 Free PMC article.
References
-
- Barker, J., T. J. Humphrey, and M. W. Brown. 1999. Survival of Escherichia coli O157 in a soil protozoan: implications for disease. FEMS Microbiol. Lett. 173:291-295. - PubMed
-
- Bengoechea, J. A., H. Najdenski, and M. Skurnik. 2004. Lipopolysaccharide O antigen status of Yersinia enterocolitica O:8 is essential for virulence and absence of O antigen affects the expression of other Yersinia virulence factors. Mol. Microbiol. 52:451-469. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

