Haemophilus ducreyi Outer membrane determinants, including DsrA, define two clonal populations
- PMID: 15784585
- PMCID: PMC1087395
- DOI: 10.1128/IAI.73.4.2387-2399.2005
Haemophilus ducreyi Outer membrane determinants, including DsrA, define two clonal populations
Abstract
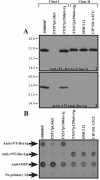
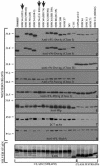
The Haemophilus ducreyi outer membrane component DsrA (for ducreyi serum resistance A) is necessary for complete resistance to normal human serum (NHS). When DsrA expression in 19 temporally and geographically diverse clinical isolates of H. ducreyi was examined by Western blotting, 5 of the strains expressed a different immunotype of the DsrA protein (DsrA(II)) than the well-characterized prototypical strain 35000HP (DsrA(I)). The predicted DsrA proteins expressed by the DsrA(II) strains were 100% identical to each other but only 48% identical to that of strain 35000HP. In addition to the DsrA(II) protein, class II strains also expressed variant forms of other outer membrane proteins (OMPs) including NcaA (necessary for collagen adhesion A), DltA (ducreyi lectin A), Hlp (H. ducreyi lipoprotein), major OMP, and/or OmpA2 (for OMP A2) and synthesized a distinct, faster-migrating lipooligosaccharide. Based on these data, strains expressing DsrA(I) were termed class I, and those expressing DsrA(II) were termed class II. Expression of dsrA(II) from strain CIP 542 ATCC in the class I dsrA(I) mutant FX517 (35000HP background), which does not express a DsrA protein, rendered this strain resistant to 50% NHS. This demonstrates that DsrA(II) protein is also critical to serum resistance. Taken together, these results indicate that there are two clonal populations of H. ducreyi. The implications of two classes of H. ducreyi strains differing in important antigenic outer membrane components are discussed.
Figures




References
-
- Alfa, M. J., and P. DeGagne. 1997. Attachment of Haemophilus ducreyi to human foreskin fibroblasts involves LOS and fibronectin. Microb. Pathog. 22:39-46 - PubMed
-
- Al-Tawfiq, J. A., K. R. Fortney, B. P. Katz, A. F. Hood, C. Elkins, and S. M. Spinola. 2000. An isogenic hemoglobin receptor-deficient mutant of Haemophilus ducreyi is attenuated in the human model of experimental infection. J. Infect. Dis. 181:1049-1054. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

