The UspA2 protein of Moraxella catarrhalis is directly involved in the expression of serum resistance
- PMID: 15784586
- PMCID: PMC1087425
- DOI: 10.1128/IAI.73.4.2400-2410.2005
The UspA2 protein of Moraxella catarrhalis is directly involved in the expression of serum resistance
Abstract
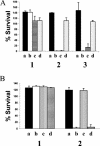
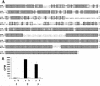
Many strains of Moraxella catarrhalis are resistant to the bactericidal activity of normal human serum. Previous studies have shown that mutations involving the insertion of an antibiotic resistance cartridge into the M. catarrhalis uspA2 gene resulted in the conversion of a serum-resistant strain to a serum-sensitive phenotype. In the present study, the deletion of the entire uspA2 gene from the serum-resistant M. catarrhalis strain O35E resulted in a serum-sensitive phenotype and did not affect either the rate of growth or the lipooligosaccharide expression profile of this mutant. Inactivation of the classical complement pathway in normal human serum with Mg2+ and EGTA resulted in the survival of this uspA2 mutant. In contrast, blocking of the alternative complement pathway did not protect this uspA2 mutant from complement-mediated killing. To determine whether the UspA2 protein is directly involved in serum resistance, transformation and allelic exchange were used to replace the uspA2 gene in the serum-resistant strain O35E with the uspA2 gene from the serum-sensitive M. catarrhalis strain MC317. The resultant O35E transformant exhibited a serum-sensitive phenotype. Similarly, when the uspA2 gene from the serum-resistant strain O35E was used to replace the uspA2 gene in the serum-sensitive strain MC317, the MC317 transformant acquired serum resistance. The use of hybrid O35E-MC317 uspA2 genes showed that the N-terminal half of the O35E protein contained a 102-amino-acid region that was involved in the expression of serum resistance. In addition, when the uspA2 genes from strains O35E and MC317 were cloned and expressed in Haemophilus influenzae DB117, only the O35E UspA2 protein caused a significant increase in the serum resistance of the H. influenzae recombinant strain. These results prove that the UspA2 protein is directly involved in the expression of serum resistance by certain M. catarrhalis strains.
Figures
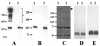



References
-
- Adlowitz, D. G., T. Hiltke, A. J. Lesse, and T. F. Murphy. 2004. Identification and characterization of outer membrane proteins G1a and G1b of Moraxella catarrhalis. Vaccine 22:2533-2540. - PubMed
-
- Alexander, H. E. 1965. The Haemophilus group, p. 724-741. In R. J. Dubos and J. G. Hirsch (ed.), Bacterial and mycotic infections of man. J. B. Lippincott Co., Philadelphia, Pa.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

