Heterologous immunity in the absence of variant-specific antibodies after exposure to subpatent infection with blood-stage malaria
- PMID: 15784594
- PMCID: PMC1087398
- DOI: 10.1128/IAI.73.4.2478-2485.2005
Heterologous immunity in the absence of variant-specific antibodies after exposure to subpatent infection with blood-stage malaria
Abstract
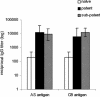
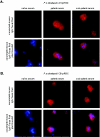
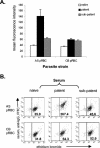
We examined immunity induced by subpatent blood-stage malaria (undetectable by microscopy) using the rodent malaria parasite, Plasmodium chabaudi chabaudi, postulating that limited infection may allow expansion of antigen-specific T cells that are normally deleted by apoptosis. After three infections drug cured at 48 h, mice were protected against high-dose challenge with homologous or heterologous parasites (different strain or variant). Immunity differed from that generated by three untreated, patent infections. Subpatently infected mice lacked immunoglobulin G (IgG) to variant surface antigens, despite producing similar titers of total malaria-specific IgG to those produced by patently infected mice, including antibodies specific for merozoite surface antigens conserved between heterologous strains. Antigen-specific proliferation of splenocytes harvested prechallenge was significantly higher in subpatently infected mice than in patently infected or naive mice. In subpatently infected mice, lymphoproliferation was similar in response to homologous and heterologous parasites, suggesting that antigenic targets of cell-mediated immunity were conserved. A Th1 cytokine response was evident during challenge. Apoptosis of CD4+ and CD8+ splenic lymphocytes occurred during patent but not subpatent infection, suggesting a reason for the relative prominence of cell-mediated immunity after subpatent infection. In conclusion, subpatent infection with blood stage malaria parasites induced protective immunity, which differed from that induced by patent infection and targeted conserved antigens. These findings suggest that alternative vaccine strategies based on delivery of multiple parasite antigens at low dose may induce effective immunity targeting conserved determinants.
Figures




References
-
- Braley-Mullen, H. 1976. Secondary IgG responses to type III pneumococcal polysaccharide. II. Different cellular requirements for induction and elicitation. J. Immunol. 116:904-910. - PubMed
-
- Branch, O. H., V. Udhayakumar, A. W. Hightower, A. J. Oloo, W. A. Hawley, B. L. Nahlen, P. B. Bloland, D. C. Kaslow, and A. A. Lal. 1998. A longitudinal investigation of IgG and IgM antibody responses to the merozoite surface protein-1 19-kilodalton domain of Plasmodium falciparum in pregnant women and infants: associations with febrile illness, parasitemia, and anemia. Am. J. Trop. Med. Hyg. 58:211-219. - PubMed
-
- Carter, R. 1978. Studies on enzyme variation in the murine malaria parasites Plasmodium berghei, P. yoelii, P. vinckei and P. chabaudi by starch gel electrophoresis. Parasitology 76:241-267. - PubMed
-
- Cohen, S., I. A. McGregor, and S. C. Carrington. 1961. Gamma-globulin and acquired immunity to human malaria. Nature 192:733-737. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

