Shiga toxin is transported from the endoplasmic reticulum following interaction with the luminal chaperone HEDJ/ERdj3
- PMID: 15784599
- PMCID: PMC1087411
- DOI: 10.1128/IAI.73.4.2524-2532.2005
Shiga toxin is transported from the endoplasmic reticulum following interaction with the luminal chaperone HEDJ/ERdj3
Abstract
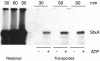
Shiga toxin (Stx) follows a complex intracellular pathway in order to kill susceptible cells. After binding to cell surface glycolipids, the toxin is internalized and trafficked in retrograde fashion to the endoplasmic reticulum (ER). From the ER lumen, the toxin must gain access to the cytoplasm, where it enzymatically inactivates the 28S rRNA, inhibiting protein synthesis. The host molecules involved in this pathway and the mechanisms utilized by the toxin to access the cytoplasm from the ER are largely unknown. We found that Stx is capable of energy-dependent transport across the ER lumen, as has recently been demonstrated for the cholera and ricin toxins. Genetic screening for molecules involved in Shiga toxin trafficking yielded a cDNA encoding a prematurely truncated protein. Characterization of this cDNA revealed that it encodes a novel Hsp40 chaperone, designated HEDJ or ERdj3, localized to the ER lumen, where it interacts with BiP, a molecule known to be involved in protein retrotranslocation out of the ER. We demonstrated that within the ER lumen Stx interacts with HEDJ and other chaperones known to be involved in retrotranslocation of proteins across the ER membrane. Moreover, sequential immunoprecipitation revealed that Shiga toxin was present in a complex that included HEDJ and Sec61, the translocon through which proteins are retrotranslocated to the cytoplasm. These findings suggest that HEDJ is a component of the ER quality control system and that Stx utilizes HEDJ and other ER-localized chaperones for transport from the ER lumen to the cytosol.
Figures


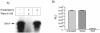
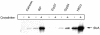

References
-
- Bies, C., S. Guth, K. Janoschek, W. Nastainczyk, J. Volkmer, and R. Zimmermann. 1999. A Scj1p homolog and folding catalysts present in dog pancreas microsomes. Biol. Chem. 380:1175-1182. - PubMed
-
- Garred, O., B. van Deurs, and K. Sandvig. 1995. Furin-induced cleavage and activation of Shiga toxin. J. Biol. Chem. 270:10817-10821. - PubMed
-
- Hazes, B., and R. J. Read. 1997. Accumulating evidence suggests that several AB-toxins subvert the endoplasmic reticulum-associated protein degradation pathway to enter target cells. Biochemistry 36:11051-11054. - PubMed
-
- Hirt, B. 1967. Selective extraction of polyoma DNA from infected mouse cell cultures. J. Mol. Biol. 26:365-369. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

