Transcriptional regulation of two stage-specifically expressed genes in the protozoan parasite Toxoplasma gondii
- PMID: 15784612
- PMCID: PMC1903550
- DOI: 10.1093/nar/gki314
Transcriptional regulation of two stage-specifically expressed genes in the protozoan parasite Toxoplasma gondii
Abstract
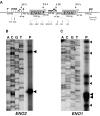
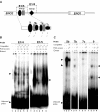
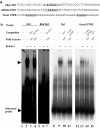
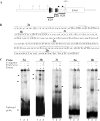
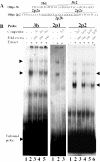
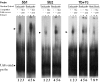
The protozoan parasite Toxoplasma gondii differentially expresses two distinct enolase isoenzymes known as ENO1 and ENO2, respectively. To understand differential gene expression during tachyzoite to bradyzoite conversion, we have characterized the two T.gondii enolase promoters. No homology could be found between these sequences and no TATA or CCAAT boxes were evident. The differential activation of the ENO1 and ENO2 promoters during tachyzoite to bradyzoite differentiation was investigated by deletion analysis of 5'-flanking regions fused to the chloramphenicol acetyltransferase reporter followed by transient transfection. Our data indicate that in proliferating tachyzoites, the repression of ENO1 involves a negative distal regulatory region (nucleotides -1245 to -625) in the promoter whereas a proximal regulatory region in the ENO2 promoter directs expression at a low level. In contrast, the promoter activity of ENO1 is highly induced following the conversion of tachyzoites into resting bradyzoites. The ENO2 promoter analysis in bradyzoites showed that there are two upstream repression sites (nucleotides -1929 to -1067 and -456 to -222). Furthermore, electrophoresis mobility shift assays demonstrated the presence of DNA-binding proteins in tachyzoite and bradyzoite nuclear lysates that bound to stress response elements (STRE), heat shock-like elements (HSE) and other cis-regulatory elements in the upstream regulatory regions of ENO1 and ENO2. Mutation of the consensus AGGGG sequence, completely abolished protein binding to an oligonucleotide containing this element. This study defines the first characterization of cis-regulatory elements and putative transcription factors involved in gene regulation of the important pathogen T.gondii.
Figures


Similar articles
-
Differential expression of two plant-like enolases with distinct enzymatic and antigenic properties during stage conversion of the protozoan parasite Toxoplasma gondii.J Mol Biol. 2001 Jun 22;309(5):1017-27. doi: 10.1006/jmbi.2001.4730. J Mol Biol. 2001. PMID: 11399076
-
Evidence for nuclear localisation of two stage-specific isoenzymes of enolase in Toxoplasma gondii correlates with active parasite replication.Int J Parasitol. 2002 Oct;32(11):1399-410. doi: 10.1016/s0020-7519(02)00129-7. Int J Parasitol. 2002. PMID: 12350375
-
Silencing of tachyzoite enolase 2 alters nuclear targeting of bradyzoite enolase 1 in Toxoplasma gondii.Microbes Infect. 2010 Jan;12(1):19-27. doi: 10.1016/j.micinf.2009.09.010. Epub 2009 Sep 19. Microbes Infect. 2010. PMID: 19770069
-
The differential expression of multiple isoenzyme forms during stage conversion of Toxoplasma gondii: an adaptive developmental strategy.Int J Parasitol. 2001 Aug;31(10):1023-31. doi: 10.1016/s0020-7519(01)00193-x. Int J Parasitol. 2001. PMID: 11429165 Review.
-
The development and biology of bradyzoites of Toxoplasma gondii.Front Biosci. 2000 Apr 1;5:D391-405. doi: 10.2741/weiss. Front Biosci. 2000. PMID: 10762601 Free PMC article. Review.
Cited by
-
The transcription of bradyzoite genes in Toxoplasma gondii is controlled by autonomous promoter elements.Mol Microbiol. 2008 Jun;68(6):1502-18. doi: 10.1111/j.1365-2958.2008.06249.x. Epub 2008 Apr 21. Mol Microbiol. 2008. PMID: 18433450 Free PMC article.
-
The transcriptome of Toxoplasma gondii.BMC Biol. 2005 Dec 2;3:26. doi: 10.1186/1741-7007-3-26. BMC Biol. 2005. PMID: 16324218 Free PMC article.
-
Putative cis-regulatory elements associated with heat shock genes activated during excystation of Cryptosporidium parvum.PLoS One. 2010 Mar 4;5(3):e9512. doi: 10.1371/journal.pone.0009512. PLoS One. 2010. PMID: 20209102 Free PMC article.
-
Nuclear glycolytic enzyme enolase of Toxoplasma gondii functions as a transcriptional regulator.PLoS One. 2014 Aug 25;9(8):e105820. doi: 10.1371/journal.pone.0105820. eCollection 2014. PLoS One. 2014. PMID: 25153525 Free PMC article.
-
Functional analysis of key nuclear trafficking components reveals an atypical Ran network required for parasite pathogenesis.Mol Microbiol. 2008 Oct;70(2):410-20. doi: 10.1111/j.1365-2958.2008.06419.x. Epub 2008 Aug 27. Mol Microbiol. 2008. PMID: 18761691 Free PMC article.
References
-
- Israelski D.M., Remington J.S. Toxoplasmosis in patients with cancer. Clin. Infect. Dis. 1993;17:423–435. - PubMed
-
- Luft B.J., Remington J.S. Toxoplasmic encephalitis in AIDS. Clin. Infect. Dis. 1992;15:211–222. - PubMed
-
- Soldati D., Boothroyd J.C. Transient transfection and expression in the obligate intracellular parasite Toxoplasma gondii. Science. 1993;260:349–352. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Miscellaneous

