In vivo dynamics of retinal injury and repair in the rhodopsin mutant dog model of human retinitis pigmentosa
- PMID: 15784735
- PMCID: PMC555975
- DOI: 10.1073/pnas.0408892102
In vivo dynamics of retinal injury and repair in the rhodopsin mutant dog model of human retinitis pigmentosa
Abstract
Genetic and environmental factors modify the severity of human neurodegenerations. Retinal degenerations caused by rhodopsin gene mutations show severity differences within and between families and even within regions of the same eye. Environmental light is thought to contribute to this variation. In the naturally occurring dog model of the human disorder, we found that modest light levels, as used in routine clinical practice, dramatically accelerated the neurodegeneration. Dynamics of acute retinal injury (consisting of abnormal intraretinal light scattering) were visualized in vivo in real time with high-resolution optical imaging. Long term consequences included fast or slow retinal degeneration or repair of injury depending on the dose of light exposure. These experiments provide a platform to study mechanisms of neuronal injury, repair, compensation, and degeneration. The data also argue for a gene-specific clinical trial of light reduction in human rhodopsin disease.
Figures
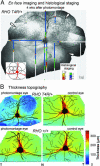


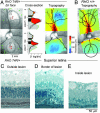

References
-
- Benn, S. C. & Woolf, C. J. (2004) Nat. Rev. Neurosci. 5, 686–700. - PubMed
-
- Pacione, L. R., Szego, M. J., Ikeda, S., Nishina, P. M. & McInnes, R. R. (2003) Annu. Rev. Neurosci. 26, 657–700. - PubMed
-
- Organisciak, D. T. & Winkler, B. S. (1994) Prog. Ret. Eye Res. 13, 1–29.
-
- Jacobson S. G. & McInnes, R. R. (2002) Nat. Genet. 32, 215–216. - PubMed
-
- Wenzel, A., Grimm, C., Samardzija, M. & Reme, C. E. (2005) Prog. Ret. Eye Res. 24, 275–306. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

