The Xenopus TACC homologue, maskin, functions in mitotic spindle assembly
- PMID: 15788567
- PMCID: PMC1142428
- DOI: 10.1091/mbc.e04-10-0926
The Xenopus TACC homologue, maskin, functions in mitotic spindle assembly
Abstract
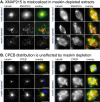
Maskin is the Xenopus homolog of the transforming acidic coiled coil (TACC)-family of microtubule and centrosome-interacting proteins. Members of this family share a approximately 200 amino acid coiled coil motif at their C-termini, but have only limited homology outside of this domain. In all species examined thus far, perturbations of TACC proteins lead to disruptions of cell cycle progression and/or embryonic lethality. In Drosophila, Caenorhabditis elegans, and humans, these disruptions have been attributed to mitotic spindle assembly defects, and the TACC proteins in these organisms are thought to function as structural components of the spindle. In contrast, cell division failure in early Xenopus embryo blastomeres has been attributed to a role of maskin in regulating the translation of, among others, cyclin B1 mRNA. In this study, we show that maskin, like other TACC proteins, plays a direct role in mitotic spindle assembly in Xenopus egg extracts and that this role is independent of cyclin B. Maskin immunodepletion and add-back experiments demonstrate that maskin, or a maskin-associated activity, is required for two distinct steps during spindle assembly in Xenopus egg extracts that can be distinguished by their response to "rescue" experiments. Defects in the "early" step, manifested by greatly reduced aster size during early time points in maskin-depleted extracts, can be rescued by readdition of purified full-length maskin. Moreover, defects in this step can also be rescued by addition of only the TACC-domain of maskin. In contrast, defects in the "late" step during spindle assembly, manifested by abnormal spindles at later time points, cannot be rescued by readdition of maskin. We show that maskin interacts with a number of proteins in egg extracts, including XMAP215, a known modulator of microtubule dynamics, and CPEB, a protein that is involved in translational regulation of important cell cycle regulators. Maskin depletion from egg extracts results in compromised microtubule asters and spindles and the mislocalization of XMAP215, but CPEB localization is unaffected. Together, these data suggest that in addition to its previously reported role as a translational regulator, maskin is also important for mitotic spindle assembly.
Figures





Similar articles
-
Function and regulation of Maskin, a TACC family protein, in microtubule growth during mitosis.J Cell Biol. 2005 Sep 26;170(7):1057-66. doi: 10.1083/jcb.200504037. Epub 2005 Sep 19. J Cell Biol. 2005. PMID: 16172207 Free PMC article.
-
Xenopus TACC3/maskin is not required for microtubule stability but is required for anchoring microtubules at the centrosome.Mol Biol Cell. 2008 Aug;19(8):3347-56. doi: 10.1091/mbc.e07-11-1204. Epub 2008 May 28. Mol Biol Cell. 2008. PMID: 18508920 Free PMC article.
-
CPEB, maskin, and cyclin B1 mRNA at the mitotic apparatus: implications for local translational control of cell division.Cell. 2000 Oct 27;103(3):435-47. doi: 10.1016/s0092-8674(00)00135-5. Cell. 2000. PMID: 11081630
-
AINT/ERIC/TACC: an expanding family of proteins with C-terminal coiled coil domains.Leuk Lymphoma. 2002 Jul;43(7):1455-9. doi: 10.1080/1042819022386644. Leuk Lymphoma. 2002. PMID: 12389629 Review.
-
Role of centrosomal adaptor proteins of the TACC family in the regulation of microtubule dynamics during mitotic cell division.Biol Chem. 2013 Nov;394(11):1411-23. doi: 10.1515/hsz-2013-0184. Biol Chem. 2013. PMID: 23787465 Review.
Cited by
-
TPX2 is required for postmitotic nuclear assembly in cell-free Xenopus laevis egg extracts.J Cell Biol. 2006 Jun 5;173(5):685-94. doi: 10.1083/jcb.200512107. Epub 2006 May 30. J Cell Biol. 2006. PMID: 16735579 Free PMC article.
-
Aurora A site specific TACC3 phosphorylation regulates astral microtubule assembly by stabilizing γ-tubulin ring complex.BMC Mol Cell Biol. 2019 Dec 10;20(1):58. doi: 10.1186/s12860-019-0242-z. BMC Mol Cell Biol. 2019. PMID: 31823729 Free PMC article.
-
Csi1p recruits alp7p/TACC to the spindle pole bodies for bipolar spindle formation.Mol Biol Cell. 2014 Sep 15;25(18):2750-60. doi: 10.1091/mbc.E14-03-0786. Epub 2014 Jul 23. Mol Biol Cell. 2014. PMID: 25057016 Free PMC article.
-
XTACC3-XMAP215 association reveals an asymmetric interaction promoting microtubule elongation.Nat Commun. 2014 Sep 29;5:5072. doi: 10.1038/ncomms6072. Nat Commun. 2014. PMID: 25262927 Free PMC article.
-
Functions and mechanisms of RNA tailing by metazoan terminal nucleotidyltransferases.Wiley Interdiscip Rev RNA. 2021 Mar;12(2):e1622. doi: 10.1002/wrna.1622. Epub 2020 Jul 22. Wiley Interdiscip Rev RNA. 2021. PMID: 33145994 Free PMC article. Review.
References
-
- Bellanger, J.-M. and Gönczy. P. (2003). TAC-1 and ZYG-9 form a complex that promotes microtubule assembly in C. elegans embryos. Curr. Biol. 13, 1488–1498. - PubMed
-
- Cassimeris, L., and Skibbens, R.V. (2003). Regulated assembly of the mitotic spindle: a perspective from two ends. Curr. Issues Mol. Biol. 5, 99–112. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

