The prion protein and its paralogue Doppel affect calcium signaling in Chinese hamster ovary cells
- PMID: 15788568
- PMCID: PMC1142425
- DOI: 10.1091/mbc.e04-10-0915
The prion protein and its paralogue Doppel affect calcium signaling in Chinese hamster ovary cells
Abstract
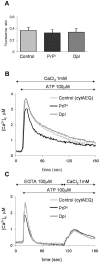
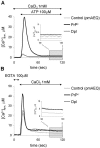
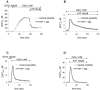
The function of the prion protein (PrP(c)), implicated in transmissible spongiform encephalopathies (TSEs), is largely unknown. We examined the possible influence of PrP(c) on Ca(2+) homeostasis, by analyzing local Ca(2+) fluctuations in cells transfected with PrP(c) and Ca(2+)-sensitive aequorin chimeras targeted to defined subcellular compartments. In agonist-stimulated cells, the presence of PrP(c) sharply increases the Ca(2+) concentration of subplasma membrane Ca(2+) domains, a feature that may explain the impairment of Ca(2+)-dependent neuronal excitability observed in TSEs. PrP(c) also limits Ca(2+) release from the endoplasmic reticulum and Ca(2+) uptake by mitochondria, thus rendering unlikely the triggering of cell death pathways. Instead, cells expressing Doppel, a PrP(c) paralogue, display opposite effects, which, however, are abolished by the coexpression of PrP(c). These findings are consistent with the functional interplay and antagonistic role attributed to the proteins, whereby PrP(c) protects, and Doppel sensitizes, cells toward stress conditions.
Figures



Similar articles
-
Cellular prion protein is implicated in the regulation of local Ca2+ movements in cerebellar granule neurons.J Neurochem. 2011 Mar;116(5):881-90. doi: 10.1111/j.1471-4159.2010.07015.x. Epub 2011 Jan 7. J Neurochem. 2011. PMID: 21214552
-
Genetic mapping of activity determinants within cellular prion proteins: N-terminal modules in PrPC offset pro-apoptotic activity of the Doppel helix B/B' region.J Biol Chem. 2004 Dec 31;279(53):55443-54. doi: 10.1074/jbc.M404794200. Epub 2004 Sep 29. J Biol Chem. 2004. PMID: 15459186
-
The cellular prion protein (PrPC) prevents apoptotic neuronal cell death and mitochondrial dysfunction induced by serum deprivation.Brain Res Mol Brain Res. 2004 Apr 29;124(1):40-50. doi: 10.1016/j.molbrainres.2004.02.005. Brain Res Mol Brain Res. 2004. PMID: 15093684
-
The prion gene complex encoding PrP(C) and Doppel: insights from mutational analysis.Gene. 2001 Sep 5;275(1):1-18. doi: 10.1016/s0378-1119(01)00627-8. Gene. 2001. PMID: 11574147 Review.
-
The highways and byways of prion protein trafficking.Trends Cell Biol. 2005 Feb;15(2):102-11. doi: 10.1016/j.tcb.2004.12.002. Trends Cell Biol. 2005. PMID: 15695097 Review.
Cited by
-
Alterations in Ca2+-buffering in prion-null mice: association with reduced afterhyperpolarizations in CA1 hippocampal neurons.J Neurosci. 2008 Apr 9;28(15):3877-86. doi: 10.1523/JNEUROSCI.0675-08.2008. J Neurosci. 2008. PMID: 18400886 Free PMC article.
-
PrPC knockdown by liposome-siRNA-peptide complexes (LSPCs) prolongs survival and normal behavior of prion-infected mice immunotolerant to treatment.PLoS One. 2019 Jul 22;14(7):e0219995. doi: 10.1371/journal.pone.0219995. eCollection 2019. PLoS One. 2019. PMID: 31329627 Free PMC article.
-
Medulla oblongata transcriptome changes during presymptomatic natural scrapie and their association with prion-related lesions.BMC Genomics. 2012 Aug 16;13:399. doi: 10.1186/1471-2164-13-399. BMC Genomics. 2012. PMID: 22897917 Free PMC article.
-
Activation of p53-regulated pro-apoptotic signaling pathways in PrP-mediated myopathy.BMC Genomics. 2009 Apr 28;10:201. doi: 10.1186/1471-2164-10-201. BMC Genomics. 2009. PMID: 19400950 Free PMC article.
-
Direct detection of soil-bound prions.PLoS One. 2007 Oct 24;2(10):e1069. doi: 10.1371/journal.pone.0001069. PLoS One. 2007. PMID: 17957252 Free PMC article.
References
-
- Arnaudeau, S., Frieden, M., Nakamura, K., Castelbou, C., Michalak, M., and Demaurex, N. (2002). Calreticulin differentially modulates calcium uptake and release in the endoplasmic reticulum and mitochondria. J. Biol. Chem. 277, 46696–46705. - PubMed
-
- Atarashi, R., Nishida, N., Shigematsu, K., Goto, S., Kondo, T., Sakaguchi, S., and Katamine, S. (2003). Deletion of N-terminal residues 23–88 from prion protein (PrP) abrogates the potential to rescue PrP-deficient mice from PrP-like protein/doppel-induced neurodegeneration. J. Biol. Chem. 278, 28944–28949. - PubMed
-
- Barrero, M. J., Montero, M., and Alvarez, J. (1997). Dynamics of [Ca2+] in the endoplasmic reticulum and cytoplasm of intact HeLa cells: a comparative study. J. Biol. Chem. 272, 27694–27699. - PubMed
-
- Barrow, P. A., Holmgren, C. D., Tapper, A. J., and Jefferys, J. G. (1999). Intrinsic physiological and morphological properties of principal cells of the hippocampus and neocortex in hamsters infected with scrapie. Neurobiol. Dis. 6, 406–423. - PubMed
-
- Behrens, A., and Aguzzi, A. (2002). Small is not beautiful: antagonizing functions for the prion protein PrP(C) and its homologue Dpl. Trends Neurosci. 25, 150–154. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

